Cellulose Polymer: History, Properties, and Environmental Impact
VerifiedAdded on 2020/03/16
|10
|2404
|360
Report
AI Summary
This report provides a comprehensive overview of cellulose polymers, beginning with a historical perspective and delving into their structure, which comprises long chains of glucose units. It explores various synthesis methods, including the extraction of cellulose from agricultural waste and wood, and its importance to society, highlighting its role in biofuels and disposable materials. The report discusses the advantages and disadvantages of cellulose, such as its good processing features and thermal insulation properties, as well as its moisture absorbency and limited thermal resistance. It also details the diverse usages of cellulose, including paper manufacturing, filtration processes, and applications in the food and textile industries. Furthermore, the report addresses the environmental impact of cellulose, emphasizing its biodegradability and potential as a sustainable alternative to synthetic polymers, concluding that the use of cellulose is beneficial for the environment. The report also includes references to relevant research papers.

POLYMERS
CELLULOSE
Student id and name
[Pick the date]
CELLULOSE
Student id and name
[Pick the date]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Contents
History of Polymer......................................................................................................................................2
Origin..........................................................................................................................................................2
Structure......................................................................................................................................................3
Synthesis.....................................................................................................................................................4
Importance to Society..................................................................................................................................4
Advantages and Disadvantages...................................................................................................................5
Usages.........................................................................................................................................................5
Environmental impact.................................................................................................................................6
Reference.....................................................................................................................................................8
1
History of Polymer......................................................................................................................................2
Origin..........................................................................................................................................................2
Structure......................................................................................................................................................3
Synthesis.....................................................................................................................................................4
Importance to Society..................................................................................................................................4
Advantages and Disadvantages...................................................................................................................5
Usages.........................................................................................................................................................5
Environmental impact.................................................................................................................................6
Reference.....................................................................................................................................................8
1

History of Polymer
The history of polymer (in general) commenced way back in 19th century whereas silica glass,
alumina and phosphoric acid re used to fill the dentures. However, the formed compounds have
significantly high solubility but very low mechanical strength. Polymers are mainly divided into
two groups natural polymer (natural rubber, cellulose, starch) and synthetic polymer (fibers,
plastics, rubbers). Nearly 80% of the polymers are termed as synthetic polymers. Polymeric
materials consists high molecular weight molecules with order of 103 - 107(Ellefesn & Tonnesen,
1971). The natural occurring polymers are cellulose, protein, resins, lignin, rubber which are
available for several centuries. Natural cellulose is the principal element of plant cell walls. It
was first recognized by Anselme Payen in 1838. In the year 1870, first derivative from cellulose
(termed as rayon) was formed by Hyatt Manufacturing Company. Further, it was chemically
synthesized by Kobhayashi and Shoda without using any enzymes in 1992. The organic
monomers of cellulose for polymer productions are mainly collected from agriculture, wood,
plant, cotton. The scientific and engineering aspects are taken into consideration in the research
of cellulose polymer formation in order to form new derivatives of polymers and with minimum
cost and as per the demand (Shoda & Sugano, 2005).
Origin
Cellulose is considered as one of the more critical and abundant occurring polymers on earth
because it has been used as main raw material for several products. The basic structure of any
plant cell walls comprises of three main elements which are highlighted below (Nishino, 2004):
33% vegetable
90% cotton
50% wood
It is essential to note that it has been found in nearly pure (98%) form in cotton fiber combined
with lignin or hemicellulose.
2
The history of polymer (in general) commenced way back in 19th century whereas silica glass,
alumina and phosphoric acid re used to fill the dentures. However, the formed compounds have
significantly high solubility but very low mechanical strength. Polymers are mainly divided into
two groups natural polymer (natural rubber, cellulose, starch) and synthetic polymer (fibers,
plastics, rubbers). Nearly 80% of the polymers are termed as synthetic polymers. Polymeric
materials consists high molecular weight molecules with order of 103 - 107(Ellefesn & Tonnesen,
1971). The natural occurring polymers are cellulose, protein, resins, lignin, rubber which are
available for several centuries. Natural cellulose is the principal element of plant cell walls. It
was first recognized by Anselme Payen in 1838. In the year 1870, first derivative from cellulose
(termed as rayon) was formed by Hyatt Manufacturing Company. Further, it was chemically
synthesized by Kobhayashi and Shoda without using any enzymes in 1992. The organic
monomers of cellulose for polymer productions are mainly collected from agriculture, wood,
plant, cotton. The scientific and engineering aspects are taken into consideration in the research
of cellulose polymer formation in order to form new derivatives of polymers and with minimum
cost and as per the demand (Shoda & Sugano, 2005).
Origin
Cellulose is considered as one of the more critical and abundant occurring polymers on earth
because it has been used as main raw material for several products. The basic structure of any
plant cell walls comprises of three main elements which are highlighted below (Nishino, 2004):
33% vegetable
90% cotton
50% wood
It is essential to note that it has been found in nearly pure (98%) form in cotton fiber combined
with lignin or hemicellulose.
2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
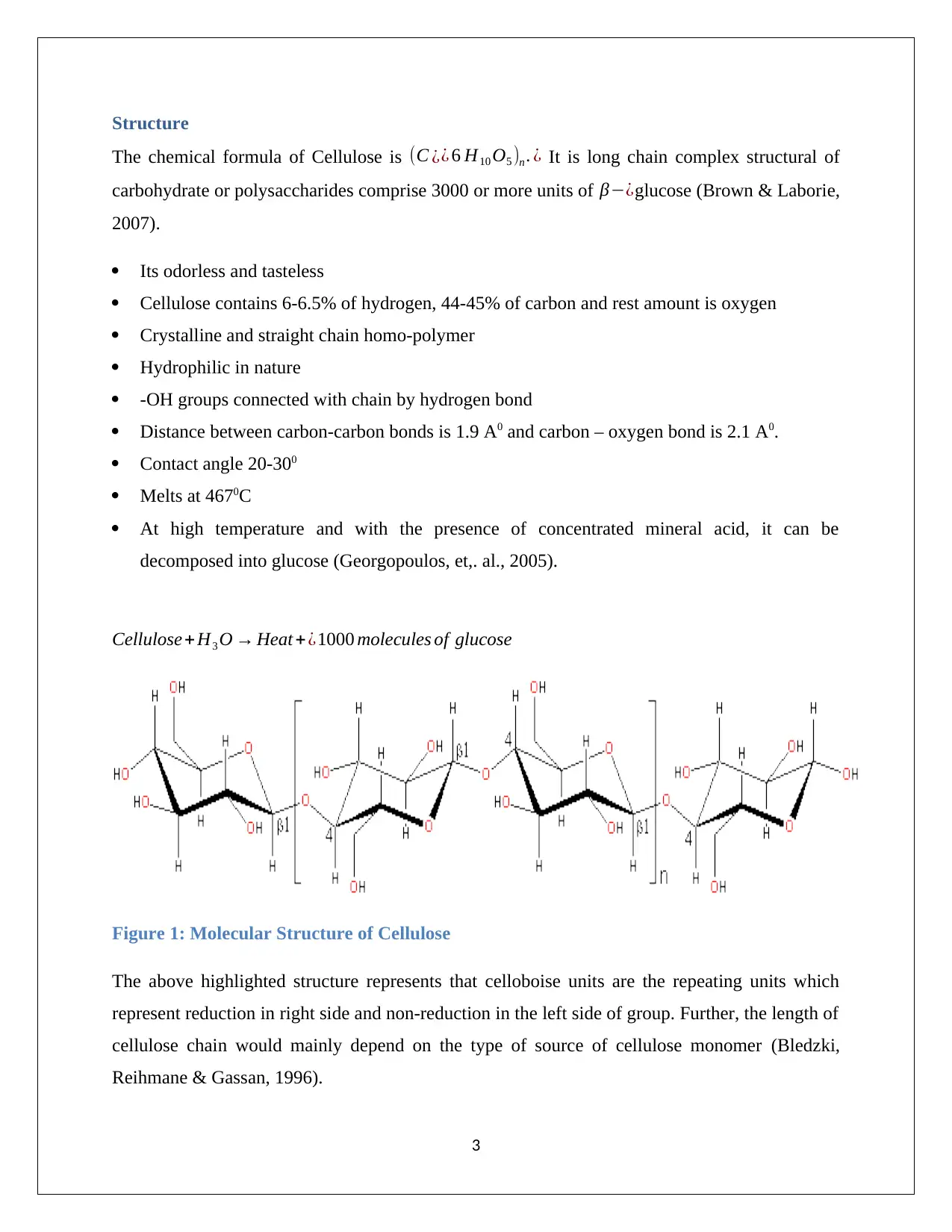
Structure
The chemical formula of Cellulose is (C ¿¿ 6 H10 O5 )n . ¿ It is long chain complex structural of
carbohydrate or polysaccharides comprise 3000 or more units of β−¿glucose (Brown & Laborie,
2007).
Its odorless and tasteless
Cellulose contains 6-6.5% of hydrogen, 44-45% of carbon and rest amount is oxygen
Crystalline and straight chain homo-polymer
Hydrophilic in nature
-OH groups connected with chain by hydrogen bond
Distance between carbon-carbon bonds is 1.9 A0 and carbon – oxygen bond is 2.1 A0.
Contact angle 20-300
Melts at 4670C
At high temperature and with the presence of concentrated mineral acid, it can be
decomposed into glucose (Georgopoulos, et,. al., 2005).
Cellulose + H3 O → Heat +¿1000 molecules of glucose
Figure 1: Molecular Structure of Cellulose
The above highlighted structure represents that celloboise units are the repeating units which
represent reduction in right side and non-reduction in the left side of group. Further, the length of
cellulose chain would mainly depend on the type of source of cellulose monomer (Bledzki,
Reihmane & Gassan, 1996).
3
The chemical formula of Cellulose is (C ¿¿ 6 H10 O5 )n . ¿ It is long chain complex structural of
carbohydrate or polysaccharides comprise 3000 or more units of β−¿glucose (Brown & Laborie,
2007).
Its odorless and tasteless
Cellulose contains 6-6.5% of hydrogen, 44-45% of carbon and rest amount is oxygen
Crystalline and straight chain homo-polymer
Hydrophilic in nature
-OH groups connected with chain by hydrogen bond
Distance between carbon-carbon bonds is 1.9 A0 and carbon – oxygen bond is 2.1 A0.
Contact angle 20-300
Melts at 4670C
At high temperature and with the presence of concentrated mineral acid, it can be
decomposed into glucose (Georgopoulos, et,. al., 2005).
Cellulose + H3 O → Heat +¿1000 molecules of glucose
Figure 1: Molecular Structure of Cellulose
The above highlighted structure represents that celloboise units are the repeating units which
represent reduction in right side and non-reduction in the left side of group. Further, the length of
cellulose chain would mainly depend on the type of source of cellulose monomer (Bledzki,
Reihmane & Gassan, 1996).
3
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Synthesis
The cellulosic material would be collected from different agriculture wastes or from wood.
The carbohydrate part of material that would not dissolve in 17.5% solution of NaOH at 200C
which is termed as ∝ - cellulose (true cellulose).
The true cellulose would be dissolved in CH3COOH and HNO3 in order to remove xylosans,
hemicellulose and lignin.
Further, the final cellulose would allow reacting with anthrone in the presence of H2SO4.
After the reaction between cellulose and anthrone, a colored compound would be made
which needs to be assayed spectrophotometrically so that the wavelength of the compound
would be nearly 635 nanometers.
The final compound would be termed as pure cellulosic from wood, agriculture and so forth
(Georgopoulos, et,. al., 2005).
There are many processes that have been taken into consideration to form different cellulosic
derivatives. Further, it is essential to note that cellulose esters and cellulose ethers are
manufactured based on heterogeneous reactions with the help of acids and the respective acid
anhydrides (March, 1994).
Importance to Society
In the modern world, it has been found that the demand for disposable materials is increasing
from various process industries. This also results in significant load on environment. Hence, the
main focus is to utilize the disposable components in such a way that would not only
environment friendly but also has some economic value to the society. In this regards, the
cellulose which is abundantly available in nature and also is a by-product of many production
units can be used to produce bio-fuels, renewable based fuels (Georgopoulos, et,. al., 2005). This
plays an imperative role to minimize the total burden of non-renewable fuels because cellulose
based bio-fuels are easier and cheaper to produce. Significant amount of fuel would be produced
from cellulose because of the unique chemical structure of cellulose. This is considered as
versatile material and superior platform to produce bio-energy that can be used for several
energy saving purposes (Rose, et. al.,2010).
4
The cellulosic material would be collected from different agriculture wastes or from wood.
The carbohydrate part of material that would not dissolve in 17.5% solution of NaOH at 200C
which is termed as ∝ - cellulose (true cellulose).
The true cellulose would be dissolved in CH3COOH and HNO3 in order to remove xylosans,
hemicellulose and lignin.
Further, the final cellulose would allow reacting with anthrone in the presence of H2SO4.
After the reaction between cellulose and anthrone, a colored compound would be made
which needs to be assayed spectrophotometrically so that the wavelength of the compound
would be nearly 635 nanometers.
The final compound would be termed as pure cellulosic from wood, agriculture and so forth
(Georgopoulos, et,. al., 2005).
There are many processes that have been taken into consideration to form different cellulosic
derivatives. Further, it is essential to note that cellulose esters and cellulose ethers are
manufactured based on heterogeneous reactions with the help of acids and the respective acid
anhydrides (March, 1994).
Importance to Society
In the modern world, it has been found that the demand for disposable materials is increasing
from various process industries. This also results in significant load on environment. Hence, the
main focus is to utilize the disposable components in such a way that would not only
environment friendly but also has some economic value to the society. In this regards, the
cellulose which is abundantly available in nature and also is a by-product of many production
units can be used to produce bio-fuels, renewable based fuels (Georgopoulos, et,. al., 2005). This
plays an imperative role to minimize the total burden of non-renewable fuels because cellulose
based bio-fuels are easier and cheaper to produce. Significant amount of fuel would be produced
from cellulose because of the unique chemical structure of cellulose. This is considered as
versatile material and superior platform to produce bio-energy that can be used for several
energy saving purposes (Rose, et. al.,2010).
4

Advantages and Disadvantages
Advantages
Cellulose has good processing features which are easy to handle and transport even for long
distance (Zimmermann, Pöhler, & Schwaller, 2005). It also results from higher yield
production process with defined and uniform quality fiber. (bio fuel) Cellulose also has good loose fitting at low cost against any structure (pipe, wiring) which is
more advantages when 40 mW /mK thermal insulation is required. (Thermal performance) This also plays a pivotal role in sound insulation process. This is cheaper and easy to handle
option as compared with other sound reduction material. (Sound reduction) Cellulose insulation is also considered as long term cost saving material (March, 1994). This also has significant utility in mold control and pest control in combination with
concentrated boric acid. Class I cellulose also provides fire retardation which is an advantageous in safety rating.
Disadvantages
Installation of cellulose insulation is difficult and time consuming. It also creats significant
with high amount of dust and also requires inadequate fixture or holes. Additionally, it
affects the working of duct by creating additional dust in the space (Georgopoulos, et,. al.,
2005).
It has been observed that loose cellulose fitting is three time in weight as compared with loss
fiberglass. Hence, additional space is required for the insulation. Cellulose also has high moisture and absorbency property which negatively impacts the
drying cost. Cellulose cannot be used for high thermal resistive application. This is also not compatible with hydrophobic polymer matrix. The cellulose is received from different plant source and hence, significant probability of
variation in quality of finished product.
Usages
Cellulose has several essential usages which are listed below:
5
Advantages
Cellulose has good processing features which are easy to handle and transport even for long
distance (Zimmermann, Pöhler, & Schwaller, 2005). It also results from higher yield
production process with defined and uniform quality fiber. (bio fuel) Cellulose also has good loose fitting at low cost against any structure (pipe, wiring) which is
more advantages when 40 mW /mK thermal insulation is required. (Thermal performance) This also plays a pivotal role in sound insulation process. This is cheaper and easy to handle
option as compared with other sound reduction material. (Sound reduction) Cellulose insulation is also considered as long term cost saving material (March, 1994). This also has significant utility in mold control and pest control in combination with
concentrated boric acid. Class I cellulose also provides fire retardation which is an advantageous in safety rating.
Disadvantages
Installation of cellulose insulation is difficult and time consuming. It also creats significant
with high amount of dust and also requires inadequate fixture or holes. Additionally, it
affects the working of duct by creating additional dust in the space (Georgopoulos, et,. al.,
2005).
It has been observed that loose cellulose fitting is three time in weight as compared with loss
fiberglass. Hence, additional space is required for the insulation. Cellulose also has high moisture and absorbency property which negatively impacts the
drying cost. Cellulose cannot be used for high thermal resistive application. This is also not compatible with hydrophobic polymer matrix. The cellulose is received from different plant source and hence, significant probability of
variation in quality of finished product.
Usages
Cellulose has several essential usages which are listed below:
5
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Cellulose is considered as one of the main constituents in paper manufacturing process.
It is having major role in the research lab because its solid state subtracts is used in thin layer
chromatography.
It is used as anticaking agent in most of the filtration processes.
Cellulose is also used as gelling agent, emulsifier and dispersing agent, insulation.
Cellulose is also used in the manufacturing of cellophane and in rayon especially for textile
industry generated from beech wood cellulose.
It is also has wide range of application as water –soluble thickener and stabilizer and binding
to the water. This property is used in the process of thickening of shampoos and conditioners.
This also enhances the ability of shampoo or soap to increase the formation of colloids
around the dirt particles (Georgopoulos, et,. al., 2005).
Microcrystalline cellulose has essential usages in food industry because of the tableting and
binding property. Cellulose also enhances the volume and texture and makes them cloudy
especially in sauces. One of critical derivative i.e. methylcellulose is used in the bakery
industry especially in the production of gluten free bakery food items (Dufresne, 2008).
The below highlighted table represents the derivative of cellulose and their use in respective
industry (March, 1994).
Derivative of cellulose Industry
Ethyl cellulose Pharmaceutical industry, paints, coating
Ehydroxylethyl cellulose Cosmetic,
Methyl cellulose Textile, tobacco, food , films industry
Carboxymethyl cellulose Paints, coating, adhesive, pharmaceutical
Cellulose xanthate Textile
Cellulose nitrate Explosive, membranes
Cellulose acetate Membranes, coating
Environmental impact
Environmentalists agree that recycling and disposal of waste material in an economic and
environment friendly manner is a pivotal challenge faced by severed countries. From empirical
researches, it can be said that the synthetic polymeric plastics requires higher than 100 years for
6
It is having major role in the research lab because its solid state subtracts is used in thin layer
chromatography.
It is used as anticaking agent in most of the filtration processes.
Cellulose is also used as gelling agent, emulsifier and dispersing agent, insulation.
Cellulose is also used in the manufacturing of cellophane and in rayon especially for textile
industry generated from beech wood cellulose.
It is also has wide range of application as water –soluble thickener and stabilizer and binding
to the water. This property is used in the process of thickening of shampoos and conditioners.
This also enhances the ability of shampoo or soap to increase the formation of colloids
around the dirt particles (Georgopoulos, et,. al., 2005).
Microcrystalline cellulose has essential usages in food industry because of the tableting and
binding property. Cellulose also enhances the volume and texture and makes them cloudy
especially in sauces. One of critical derivative i.e. methylcellulose is used in the bakery
industry especially in the production of gluten free bakery food items (Dufresne, 2008).
The below highlighted table represents the derivative of cellulose and their use in respective
industry (March, 1994).
Derivative of cellulose Industry
Ethyl cellulose Pharmaceutical industry, paints, coating
Ehydroxylethyl cellulose Cosmetic,
Methyl cellulose Textile, tobacco, food , films industry
Carboxymethyl cellulose Paints, coating, adhesive, pharmaceutical
Cellulose xanthate Textile
Cellulose nitrate Explosive, membranes
Cellulose acetate Membranes, coating
Environmental impact
Environmentalists agree that recycling and disposal of waste material in an economic and
environment friendly manner is a pivotal challenge faced by severed countries. From empirical
researches, it can be said that the synthetic polymeric plastics requires higher than 100 years for
6
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

decomposed (Pothan, et.al., 2007). The new mission in this regards can be viewed as shown
below:
“Standard input materials currently used in the plastics industry have been almost completely
petro-chemical based. Companies are now seeking to substitute petroleum-based products, like
plastics and polymers, with sustainable raw materials.”
The substitutes can be natural polymers such as cellulose. This is because the current research
suggests that cellulose polymer can be treated as environmentally friendly cellulosic derivative
which can be utilized for biofuels after their disposal. In this regards, the example of strata Plast
C plastics can be taken into consideration which is a newly generated derivative from cellulose
polymer. This plastic is considerably low in cost, easy to operate, and has high durability, high
strength and can be decomposed in 7-8 weeks (Bledzki & Gassan, 1999). After discussing the
product life cycle analysis (LCA) of various derivative of cellulose, it has been found that
cellulose has low environmental impact. Further, after the decomposition of this cellulosic plastic
(strata Plast C plastics), the decomposed material would be sent back to the soil which are
returns a part of nutrient back to earth. Moreover, many of the material synthesized from natural
cellulose polymer would be bio-degradable and thus, this would be termed as an excellent aspect
for the environment. Therefore, it can be concluded that that use of cellulose instead of other
synthetic polymers would be positive for the environmental (Wang, et.al., 2007).
7
below:
“Standard input materials currently used in the plastics industry have been almost completely
petro-chemical based. Companies are now seeking to substitute petroleum-based products, like
plastics and polymers, with sustainable raw materials.”
The substitutes can be natural polymers such as cellulose. This is because the current research
suggests that cellulose polymer can be treated as environmentally friendly cellulosic derivative
which can be utilized for biofuels after their disposal. In this regards, the example of strata Plast
C plastics can be taken into consideration which is a newly generated derivative from cellulose
polymer. This plastic is considerably low in cost, easy to operate, and has high durability, high
strength and can be decomposed in 7-8 weeks (Bledzki & Gassan, 1999). After discussing the
product life cycle analysis (LCA) of various derivative of cellulose, it has been found that
cellulose has low environmental impact. Further, after the decomposition of this cellulosic plastic
(strata Plast C plastics), the decomposed material would be sent back to the soil which are
returns a part of nutrient back to earth. Moreover, many of the material synthesized from natural
cellulose polymer would be bio-degradable and thus, this would be termed as an excellent aspect
for the environment. Therefore, it can be concluded that that use of cellulose instead of other
synthetic polymers would be positive for the environmental (Wang, et.al., 2007).
7

Reference
Bledzki, A., Reihmane, K. & Gassan, J. (1996) “Properties and modification methods for
vegetable fibers for natural fiber composites,” Journal of Applied Polymer Science, vol.
59, no. 8, pp. 1329–1336.
Bledzki, K. & Gassan, J. (1999)“Composites reinforced with cellulose based fibres,” Progress
in Polymer Science, vol. 24, no. 2, pp. 221–274. )
Brown E. & Laborie, M. (2007) “Bioengineering bacterial cellulose/poly(ethylene oxide)
nanocomposites,” Biomacromolecules, vol. 8, no. 10, pp. 3074–3081.
Dufresne, A. (2008) “Polysaccharide nanocrystals reinforced nanocomposites,” Canadian
Journal of Chemistry, vol. 86, pp. 484–494.
Ellefesn, O. & Tonnesen, B. (1971) Cellulose and Cellulose Derivatives Part IV. (5th ed.). New
York: John Wiley & Sons.
Georgopoulos, S., Tarantili, E. Avgerinos, A. Andreopoulos, & Koukios, E. (2005)
“Thermoplastic polymers reinforced with fibrous agricultural residues,” Polymer
Degradation and Stability, vol. 90, no. 2, pp. 303–312.
Nishino, T. (2004). “Natural fiber sources,” in Green Composites: Polymer Composites and the
Environment. Fla: CRC Press, Boca Raton.
Pothan, L., George, C. Jacob, M. & Thomas, S. (2007) “Effect of chemical modification on the
mechanical and electrical properties of banana fiber polyester composites,” Journal of
Composite Materials, vol. 41, no. 19, pp. 2371–2386.
Rosa, M, Medeiros, E. Malmonge J. et al., (2010) “Cellulose nanowhiskers from coconut husk
fibers: effect of preparation conditions on their thermal and morphological
behavior,” Carbohydrate Polymers, vol. 81, no. 1, pp. 83–92.
Shoda M. & Sugano, Y. (2005) “Recent advances in bacterial cellulose
production,” Biotechnology and Bioprocess Engineering, vol. 10, no. 1, pp. 1–8. March,
J. (1994) Advantages Organic Chemistry. (75th ed.). New York: John Wiley & Sons.
8
Bledzki, A., Reihmane, K. & Gassan, J. (1996) “Properties and modification methods for
vegetable fibers for natural fiber composites,” Journal of Applied Polymer Science, vol.
59, no. 8, pp. 1329–1336.
Bledzki, K. & Gassan, J. (1999)“Composites reinforced with cellulose based fibres,” Progress
in Polymer Science, vol. 24, no. 2, pp. 221–274. )
Brown E. & Laborie, M. (2007) “Bioengineering bacterial cellulose/poly(ethylene oxide)
nanocomposites,” Biomacromolecules, vol. 8, no. 10, pp. 3074–3081.
Dufresne, A. (2008) “Polysaccharide nanocrystals reinforced nanocomposites,” Canadian
Journal of Chemistry, vol. 86, pp. 484–494.
Ellefesn, O. & Tonnesen, B. (1971) Cellulose and Cellulose Derivatives Part IV. (5th ed.). New
York: John Wiley & Sons.
Georgopoulos, S., Tarantili, E. Avgerinos, A. Andreopoulos, & Koukios, E. (2005)
“Thermoplastic polymers reinforced with fibrous agricultural residues,” Polymer
Degradation and Stability, vol. 90, no. 2, pp. 303–312.
Nishino, T. (2004). “Natural fiber sources,” in Green Composites: Polymer Composites and the
Environment. Fla: CRC Press, Boca Raton.
Pothan, L., George, C. Jacob, M. & Thomas, S. (2007) “Effect of chemical modification on the
mechanical and electrical properties of banana fiber polyester composites,” Journal of
Composite Materials, vol. 41, no. 19, pp. 2371–2386.
Rosa, M, Medeiros, E. Malmonge J. et al., (2010) “Cellulose nanowhiskers from coconut husk
fibers: effect of preparation conditions on their thermal and morphological
behavior,” Carbohydrate Polymers, vol. 81, no. 1, pp. 83–92.
Shoda M. & Sugano, Y. (2005) “Recent advances in bacterial cellulose
production,” Biotechnology and Bioprocess Engineering, vol. 10, no. 1, pp. 1–8. March,
J. (1994) Advantages Organic Chemistry. (75th ed.). New York: John Wiley & Sons.
8
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Wambua, J. Ivens, & Verpoest, I. (2003) “Natural fibres: can they replace glass in fibre
reinforced plastics?” Composites Science and Technology, vol. 63, no. 9, pp. 1259–1264.
(Wambua, Ivens, & Verpoest, 2003)
Wang, B., Panigrahi, S. Tabil, L. & Crerar, W. (2007) “Pre-treatment of flax fibers for use in
rotationally molded biocomposites,” Journal of Reinforced Plastics and Composites, vol.
26, no. 5, pp. 447–46.
Zimmermann, T. Pöhler, E.& Schwaller, P. (2005) “Mechanical and morphological properties
of cellulose fibril reinforced nanocomposites,” Advanced Engineering Materials, vol. 7,
no. 12, pp. 1156–1161.
9
reinforced plastics?” Composites Science and Technology, vol. 63, no. 9, pp. 1259–1264.
(Wambua, Ivens, & Verpoest, 2003)
Wang, B., Panigrahi, S. Tabil, L. & Crerar, W. (2007) “Pre-treatment of flax fibers for use in
rotationally molded biocomposites,” Journal of Reinforced Plastics and Composites, vol.
26, no. 5, pp. 447–46.
Zimmermann, T. Pöhler, E.& Schwaller, P. (2005) “Mechanical and morphological properties
of cellulose fibril reinforced nanocomposites,” Advanced Engineering Materials, vol. 7,
no. 12, pp. 1156–1161.
9
1 out of 10
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





![Analysis of Carbohydrates in Human Body: Biology Report, [Date]](/_next/image/?url=https%3A%2F%2Fdesklib.com%2Fmedia%2Fimages%2Fnh%2F7b5ea273413144b985b8efdebb047dc4.jpg&w=256&q=75)