Reverse Phase Liquid Chromatography
VerifiedAdded on 2023/03/17
|10
|2030
|86
AI Summary
This article provides an overview of reverse phase liquid chromatography, a chromatographic technique used to separate nonpolar molecules. It discusses the theory of separation, historical development, applications in various industries, advantages, and disadvantages. Find study material and solved assignments on Desklib.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

REVERSE PHASE CHROMATOGRAPHY1
REVERSE PHASE LIQUID CHROMATOGRAPHY
By (Name)
Course
Tutor
Learning institution
Date
REVERSE PHASE LIQUID CHROMATOGRAPHY
By (Name)
Course
Tutor
Learning institution
Date
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

REVERSE PHASE CHROMATOGRAPHY2
Table of Content
Introduction………………………………………………………………………………………3
Discussion …………………………………………………………………………………….4
Conclusion………………………………………………………………………………………..8
Bibliography……………………………………………………………………………………..9
Table of Content
Introduction………………………………………………………………………………………3
Discussion …………………………………………………………………………………….4
Conclusion………………………………………………………………………………………..8
Bibliography……………………………………………………………………………………..9

REVERSE PHASE CHROMATOGRAPHY3
Introduction
Reverse phase liquid chromatography is Reverse phase chromatomatagraphic technique
that separate nonpolar molecules in a solution. Nonpolar solvents such as nitrile are used as the
stationary phase used is nonpolar while the polar solvents are the moving phase (Gocan,
Cimpan, and Comer, 2016,p.90). While in the normal phase the theory of separation of particles
is based on the interaction between the polar matrix (silica beads) and the nonpolar mobile phase
(nonpolar solvent).It is viewed to be superior to the other modes of chromatographic techniques,
such as the thin layer chromatography, paper chromatography, gas chromatography among many
different methods as it can analyze a variety of target compounds.
Introduction
Reverse phase liquid chromatography is Reverse phase chromatomatagraphic technique
that separate nonpolar molecules in a solution. Nonpolar solvents such as nitrile are used as the
stationary phase used is nonpolar while the polar solvents are the moving phase (Gocan,
Cimpan, and Comer, 2016,p.90). While in the normal phase the theory of separation of particles
is based on the interaction between the polar matrix (silica beads) and the nonpolar mobile phase
(nonpolar solvent).It is viewed to be superior to the other modes of chromatographic techniques,
such as the thin layer chromatography, paper chromatography, gas chromatography among many
different methods as it can analyze a variety of target compounds.

REVERSE PHASE CHROMATOGRAPHY4
Discussion
Reverse phase liquid chromatography has gone under a series of historical development
to the present form in which nonpolar solvents are the surface of separation while polar solvents
mostly water used as the moving phase. Reverse phase liquid chromatography originated from
the field of chemical science in the 1950s and got an important role in various sectors in
chemistry such biochemistry as well as organic chemistry. In inorganic. It was also employed by
the clinical chemistry for the rapid separation, separation and analysis of drugs in the laboratory.
Reverse phase liquid chromatography was proposed to be used in the identification of
Mycobacterium by around 1989 and fully put to force as a standard test for identification of
compounds in the early -1990s (Gocan, Cimpan, and Comer, 2016,p.90). It was found to be very
advantageous compared to the slow and time consuming traditional method used in the past. A
new technique that use a pump to pump out the suspended sample components to go through the
separation phase was introduced around the 1970s.The observed peaks during analysis were
detected and sorted out into various constituents that depended on the retention time, elution rate
as well as emergency.
The theory of separation is based on the schematic diagram below, in which the
solute with both the hydrophobic and hydrophilic ends binds with the hydrophilic molecules
(stationary phase ) in the polar solvent (Mallik, Qiu, Takafuji, and Ihara,2018,p.70). This
arrangement occurs as a result of the solute molecule possessing the hydrophobic part and
binding through the hydrophobic regions to the hydrophobic stationary phase. A buffer is always
used to increase the hydrophobicity of the stationary phase to dissociate the bound molecule at a
point of the interaction between the exposed groups and the stationary phase. The particles were
released from the stationary phase, and the elute if and only If the hydrophobic interaction
Discussion
Reverse phase liquid chromatography has gone under a series of historical development
to the present form in which nonpolar solvents are the surface of separation while polar solvents
mostly water used as the moving phase. Reverse phase liquid chromatography originated from
the field of chemical science in the 1950s and got an important role in various sectors in
chemistry such biochemistry as well as organic chemistry. In inorganic. It was also employed by
the clinical chemistry for the rapid separation, separation and analysis of drugs in the laboratory.
Reverse phase liquid chromatography was proposed to be used in the identification of
Mycobacterium by around 1989 and fully put to force as a standard test for identification of
compounds in the early -1990s (Gocan, Cimpan, and Comer, 2016,p.90). It was found to be very
advantageous compared to the slow and time consuming traditional method used in the past. A
new technique that use a pump to pump out the suspended sample components to go through the
separation phase was introduced around the 1970s.The observed peaks during analysis were
detected and sorted out into various constituents that depended on the retention time, elution rate
as well as emergency.
The theory of separation is based on the schematic diagram below, in which the
solute with both the hydrophobic and hydrophilic ends binds with the hydrophilic molecules
(stationary phase ) in the polar solvent (Mallik, Qiu, Takafuji, and Ihara,2018,p.70). This
arrangement occurs as a result of the solute molecule possessing the hydrophobic part and
binding through the hydrophobic regions to the hydrophobic stationary phase. A buffer is always
used to increase the hydrophobicity of the stationary phase to dissociate the bound molecule at a
point of the interaction between the exposed groups and the stationary phase. The particles were
released from the stationary phase, and the elute if and only If the hydrophobic interaction
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
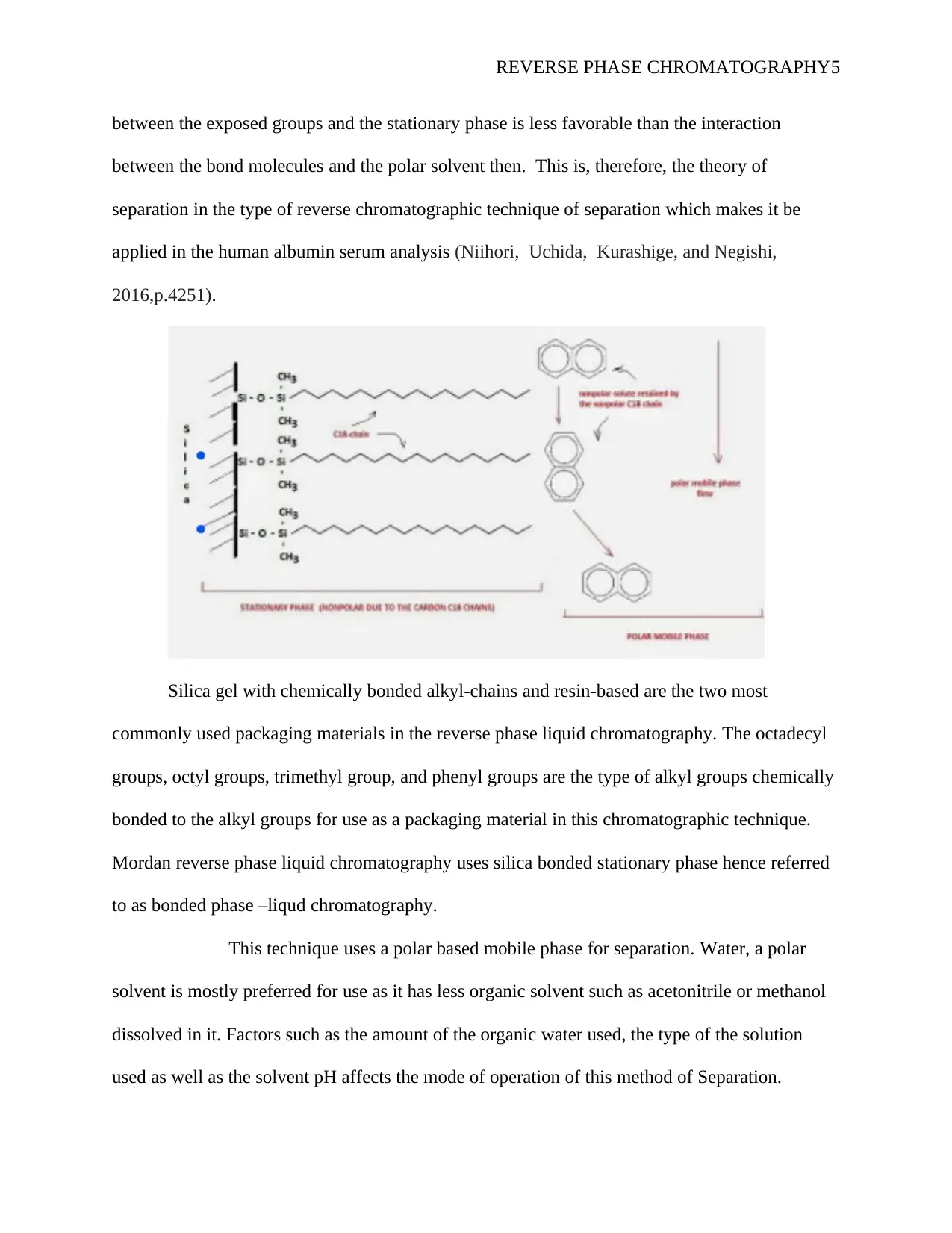
REVERSE PHASE CHROMATOGRAPHY5
between the exposed groups and the stationary phase is less favorable than the interaction
between the bond molecules and the polar solvent then. This is, therefore, the theory of
separation in the type of reverse chromatographic technique of separation which makes it be
applied in the human albumin serum analysis (Niihori, Uchida, Kurashige, and Negishi,
2016,p.4251).
Silica gel with chemically bonded alkyl-chains and resin-based are the two most
commonly used packaging materials in the reverse phase liquid chromatography. The octadecyl
groups, octyl groups, trimethyl group, and phenyl groups are the type of alkyl groups chemically
bonded to the alkyl groups for use as a packaging material in this chromatographic technique.
Mordan reverse phase liquid chromatography uses silica bonded stationary phase hence referred
to as bonded phase –liqud chromatography.
This technique uses a polar based mobile phase for separation. Water, a polar
solvent is mostly preferred for use as it has less organic solvent such as acetonitrile or methanol
dissolved in it. Factors such as the amount of the organic water used, the type of the solution
used as well as the solvent pH affects the mode of operation of this method of Separation.
between the exposed groups and the stationary phase is less favorable than the interaction
between the bond molecules and the polar solvent then. This is, therefore, the theory of
separation in the type of reverse chromatographic technique of separation which makes it be
applied in the human albumin serum analysis (Niihori, Uchida, Kurashige, and Negishi,
2016,p.4251).
Silica gel with chemically bonded alkyl-chains and resin-based are the two most
commonly used packaging materials in the reverse phase liquid chromatography. The octadecyl
groups, octyl groups, trimethyl group, and phenyl groups are the type of alkyl groups chemically
bonded to the alkyl groups for use as a packaging material in this chromatographic technique.
Mordan reverse phase liquid chromatography uses silica bonded stationary phase hence referred
to as bonded phase –liqud chromatography.
This technique uses a polar based mobile phase for separation. Water, a polar
solvent is mostly preferred for use as it has less organic solvent such as acetonitrile or methanol
dissolved in it. Factors such as the amount of the organic water used, the type of the solution
used as well as the solvent pH affects the mode of operation of this method of Separation.

REVERSE PHASE CHROMATOGRAPHY6
Reverse phase chromatography uses acetonitrile and methanol mostly as the organic solvents for
the moving phase. The selectivity for the solutions depends on the nature of the added water
(Contrepois, Jiang, and Snyder, 2015,p.1685). Such factors include the dipole moments,
solutions with more massive dipole moments such as methylene chloride, interact with the solute
with more massive dipole moments such as the nitriles, amines, and the nitro compounds. The
rate of proton donation also determine the selectivity of the solvent added, solvents with better
proton donation such as water chloroform would interact best with primary solutes such as amine
and sulfoxides.
The application of the reverse phase liquid chromatography is generally based on the
fact that both the mobile phase and the stationary phase are polar (Niihori, Uchida, Kurashige,
and Negishi, 2016,p.4251). This makes this method very applicable in the separation and
evaluation of polar molecules that are either insoluble in an organic solvent or are firmly
attached to the polar stationary phase.
Reverse phase liquid chromatography is employed in the pharmaceutical industries for
the separation and identification of compounds such as vitamins and steroids. Reverse phase
liquid chromatography is used in both food and beverage industries for the analysis of the
sweeteners, food additives, and carbohydrates (Ovčačíková, Lísa, Cífková, and Holčapek,
2016,p.80). It is also employed in the chemical industry for polymeric analysis of various types
of polymer additives. The field of environmental monitoring also employs the use of reverse
phase liquid chromatography for the study of pesticides and herbicides. In clinical analysis,
reverse liquid phase chromatography is used for the research and the determination of
catecholamines.
Reverse phase chromatography uses acetonitrile and methanol mostly as the organic solvents for
the moving phase. The selectivity for the solutions depends on the nature of the added water
(Contrepois, Jiang, and Snyder, 2015,p.1685). Such factors include the dipole moments,
solutions with more massive dipole moments such as methylene chloride, interact with the solute
with more massive dipole moments such as the nitriles, amines, and the nitro compounds. The
rate of proton donation also determine the selectivity of the solvent added, solvents with better
proton donation such as water chloroform would interact best with primary solutes such as amine
and sulfoxides.
The application of the reverse phase liquid chromatography is generally based on the
fact that both the mobile phase and the stationary phase are polar (Niihori, Uchida, Kurashige,
and Negishi, 2016,p.4251). This makes this method very applicable in the separation and
evaluation of polar molecules that are either insoluble in an organic solvent or are firmly
attached to the polar stationary phase.
Reverse phase liquid chromatography is employed in the pharmaceutical industries for
the separation and identification of compounds such as vitamins and steroids. Reverse phase
liquid chromatography is used in both food and beverage industries for the analysis of the
sweeteners, food additives, and carbohydrates (Ovčačíková, Lísa, Cífková, and Holčapek,
2016,p.80). It is also employed in the chemical industry for polymeric analysis of various types
of polymer additives. The field of environmental monitoring also employs the use of reverse
phase liquid chromatography for the study of pesticides and herbicides. In clinical analysis,
reverse liquid phase chromatography is used for the research and the determination of
catecholamines.

REVERSE PHASE CHROMATOGRAPHY7
The use of reverse phase liquid chromatography has several advantages associated
with it as compared to the other chromatographic techniques used for the same purpose. The use
of water as the moving phase possibly reduces the analyte skewed. Also, the application of
different hydrophilic solvents in normal phase liquid chromatography technique having differing
reactions compression complicates the accuracy of the gradient separations. This further
complicates the whole process due to the differences in the UV cut-off points. Reverse phase
liquid chromatography has however solved these disadvantages by only using versatile water-
based solvents (Ovčačíková, Lísa, Cífková, and Holčapek, 2016,p.80).T he uses of water-based
solutions as the moving phase in the reverse liquid chromatography makes the whole process of
separation utilizing this technique of separation cheaper and cost-effective.
This chromatographic technique is very flexible. Flexibility is the main
disadvantage of using this form of liquid chromatography as opposed to the normal phase
chromatography. This is as a result of the use of hydrophobic incorporated with non-polar, polar
ionic and ionizable solvents as the stationary phase to separate their various component, hence,
reverse phase liquid chromatography has a broader choice for the stationary phase to use than the
normal phase liquid chromatography.RPLC is extremely quick and efficient as compared to
other techniques such as thin layer chromatography (Ovčačíková, Lísa, Cífková, and Holčapek,
2016,p.80). Despite these several advantages associated with this chromatographic technique,
reverse phase liquid chromatography is costly since it requires more substantial quantities of
expensive solvents to be used as the mobile phase. Moreover, it is very complex and requires
advanced skills to run and operate as compared to other more straightforward techniques such as
thin layer chromatography.
The use of reverse phase liquid chromatography has several advantages associated
with it as compared to the other chromatographic techniques used for the same purpose. The use
of water as the moving phase possibly reduces the analyte skewed. Also, the application of
different hydrophilic solvents in normal phase liquid chromatography technique having differing
reactions compression complicates the accuracy of the gradient separations. This further
complicates the whole process due to the differences in the UV cut-off points. Reverse phase
liquid chromatography has however solved these disadvantages by only using versatile water-
based solvents (Ovčačíková, Lísa, Cífková, and Holčapek, 2016,p.80).T he uses of water-based
solutions as the moving phase in the reverse liquid chromatography makes the whole process of
separation utilizing this technique of separation cheaper and cost-effective.
This chromatographic technique is very flexible. Flexibility is the main
disadvantage of using this form of liquid chromatography as opposed to the normal phase
chromatography. This is as a result of the use of hydrophobic incorporated with non-polar, polar
ionic and ionizable solvents as the stationary phase to separate their various component, hence,
reverse phase liquid chromatography has a broader choice for the stationary phase to use than the
normal phase liquid chromatography.RPLC is extremely quick and efficient as compared to
other techniques such as thin layer chromatography (Ovčačíková, Lísa, Cífková, and Holčapek,
2016,p.80). Despite these several advantages associated with this chromatographic technique,
reverse phase liquid chromatography is costly since it requires more substantial quantities of
expensive solvents to be used as the mobile phase. Moreover, it is very complex and requires
advanced skills to run and operate as compared to other more straightforward techniques such as
thin layer chromatography.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

REVERSE PHASE CHROMATOGRAPHY8
Conclusion
In quick summary, liquid chromatography is in its current form of development as a
reverse phase liquid chromatography that uses water-based solvents as the moving phase and the
hydrophobic-polar solutions such as nitrile as the stationary phase. The difference in the
hydrophobic interaction between the exposed groups and the stationary phase in terms of the
bond strength and the interaction between the bond molecules and the polar solvent is the basic
theory of separation in reverse phase chromatography (Ovčačíková, Lísa, Cífková, and
Holčapek, 2016,p.80). RPLC has as several applications such as its application in the
pharmaceutical industries for the separation and identification of compounds such as vitamins
and steroids among so many other applications. Finally, the technique also has several
advantages and disadvantages associated with it as compared to the other methods used for the
same process of separation.
Conclusion
In quick summary, liquid chromatography is in its current form of development as a
reverse phase liquid chromatography that uses water-based solvents as the moving phase and the
hydrophobic-polar solutions such as nitrile as the stationary phase. The difference in the
hydrophobic interaction between the exposed groups and the stationary phase in terms of the
bond strength and the interaction between the bond molecules and the polar solvent is the basic
theory of separation in reverse phase chromatography (Ovčačíková, Lísa, Cífková, and
Holčapek, 2016,p.80). RPLC has as several applications such as its application in the
pharmaceutical industries for the separation and identification of compounds such as vitamins
and steroids among so many other applications. Finally, the technique also has several
advantages and disadvantages associated with it as compared to the other methods used for the
same process of separation.

REVERSE PHASE CHROMATOGRAPHY9
Bibliography
Baert, M., Martens, S., Desmet, G., de Villiers, A., Du Prez, F. and Lynn, F., 2018. Enhancing
the Possibilities of Comprehensive Two-Dimensional Liquid Chromatography through
Hyphenation of Purely Aqueous Temperature-Responsive and Reversed-Phase Liquid
Chromatography. Analytical Chemistry, 90(8), pp.4961-4967.
Contrepois, K., Jiang, L. and Snyder, M., 2015. Optimized analytical procedures for the
untargeted metabolomic profiling of human urine and plasma by combining hydrophilic
interaction (HILIC) and reverse-phase liquid chromatography (RPLC)–mass
spectrometry. Molecular & Cellular Proteomics, 14(6), pp.1684-1695.
Gupta, V., Talebi, M., Deverell, J., Sandron, S., Nesterenko, P.N., Heery, B., Thompson, F.,
Beirne, S., Wallace, G.G. and Paull, B., 2016. 3D printed titanium micro-bore columns
containing polymer monoliths for reversed-phase liquid chromatography. Analytica
Chimica Acta, 910, pp.84-94.
Gocan, S., Cimpan, G. and Comer, J., 2016. Lipophilicity measurements by liquid
chromatography. In Advances in chromatography (pp. 82-179). CRC Press
Liang, T., Fu, Q., Shen, A., Wang, H., Jin, Y., Xin, H., Ke, Y., Guo, Z. and Liang, X., 2015.
Preparation and chromatographic evaluation of a newly designed steviol glycoside
modified-silica stationary phase in hydrophilic interaction liquid chromatography and
reversed-phase liquid chromatography. Journal of Chromatography A, 1388, pp.110-118.
.
Bibliography
Baert, M., Martens, S., Desmet, G., de Villiers, A., Du Prez, F. and Lynn, F., 2018. Enhancing
the Possibilities of Comprehensive Two-Dimensional Liquid Chromatography through
Hyphenation of Purely Aqueous Temperature-Responsive and Reversed-Phase Liquid
Chromatography. Analytical Chemistry, 90(8), pp.4961-4967.
Contrepois, K., Jiang, L. and Snyder, M., 2015. Optimized analytical procedures for the
untargeted metabolomic profiling of human urine and plasma by combining hydrophilic
interaction (HILIC) and reverse-phase liquid chromatography (RPLC)–mass
spectrometry. Molecular & Cellular Proteomics, 14(6), pp.1684-1695.
Gupta, V., Talebi, M., Deverell, J., Sandron, S., Nesterenko, P.N., Heery, B., Thompson, F.,
Beirne, S., Wallace, G.G. and Paull, B., 2016. 3D printed titanium micro-bore columns
containing polymer monoliths for reversed-phase liquid chromatography. Analytica
Chimica Acta, 910, pp.84-94.
Gocan, S., Cimpan, G. and Comer, J., 2016. Lipophilicity measurements by liquid
chromatography. In Advances in chromatography (pp. 82-179). CRC Press
Liang, T., Fu, Q., Shen, A., Wang, H., Jin, Y., Xin, H., Ke, Y., Guo, Z. and Liang, X., 2015.
Preparation and chromatographic evaluation of a newly designed steviol glycoside
modified-silica stationary phase in hydrophilic interaction liquid chromatography and
reversed-phase liquid chromatography. Journal of Chromatography A, 1388, pp.110-118.
.

REVERSE PHASE CHROMATOGRAPHY10
Niihori, Y., Uchida, C., Kurashige, W. and Negishi, Y., 2016. High-resolution separation of
thiolate-protected gold clusters by reversed-phase high-performance liquid
chromatography. Physical Chemistry Chemical Physics, 18(6), pp.4251-4265.
Mallik, A.K., Qiu, H., Takafuji, M. and Ihara, H., 2018. High molecular-shape-selective
stationary phases for reversed-phase liquid chromatography: A review. TrAC Trends in
Analytical Chemistry.
Ovčačíková, M., Lísa, M., Cífková, E. and Holčapek, M., 2016. Retention behavior of lipids in
reversed-phase ultrahigh-performance liquid chromatography–electrospray ionization
mass spectrometry. Journal of Chromatography A, 1450, pp.76-85.
Shen, Y., Tolić, N., Piehowski, P.D., Shukla, A.K., Kim, S., Zhao, R., Qu, Y., Robinson, E.,
Smith, R.D. and Paša-Tolić, L., 2017. High-resolution ultrahigh-pressure long column
reversed-phase liquid chromatography for top-down proteomics. Journal of
Chromatography A, 1498, pp.99-110.
Venkatramani, C.J., Al-Sayah, M., Li, G., Goel, M., Girotti, J., Zang, L., Wigman, L., Yehl, P.
and Chetwyn, N., 2016. Simultaneous achiral-chiral analysis of pharmaceutical
compounds using two-dimensional reversed phase liquid chromatography-supercritical
fluid chromatography. Talanta, 148, pp.548-555
Niihori, Y., Uchida, C., Kurashige, W. and Negishi, Y., 2016. High-resolution separation of
thiolate-protected gold clusters by reversed-phase high-performance liquid
chromatography. Physical Chemistry Chemical Physics, 18(6), pp.4251-4265.
Mallik, A.K., Qiu, H., Takafuji, M. and Ihara, H., 2018. High molecular-shape-selective
stationary phases for reversed-phase liquid chromatography: A review. TrAC Trends in
Analytical Chemistry.
Ovčačíková, M., Lísa, M., Cífková, E. and Holčapek, M., 2016. Retention behavior of lipids in
reversed-phase ultrahigh-performance liquid chromatography–electrospray ionization
mass spectrometry. Journal of Chromatography A, 1450, pp.76-85.
Shen, Y., Tolić, N., Piehowski, P.D., Shukla, A.K., Kim, S., Zhao, R., Qu, Y., Robinson, E.,
Smith, R.D. and Paša-Tolić, L., 2017. High-resolution ultrahigh-pressure long column
reversed-phase liquid chromatography for top-down proteomics. Journal of
Chromatography A, 1498, pp.99-110.
Venkatramani, C.J., Al-Sayah, M., Li, G., Goel, M., Girotti, J., Zang, L., Wigman, L., Yehl, P.
and Chetwyn, N., 2016. Simultaneous achiral-chiral analysis of pharmaceutical
compounds using two-dimensional reversed phase liquid chromatography-supercritical
fluid chromatography. Talanta, 148, pp.548-555
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.



