Wiltshire College, Cell Biology: The Structure of the Cell Report
VerifiedAdded on 2020/03/16
|14
|2919
|55
Report
AI Summary
This report provides a comprehensive overview of cell biology, covering cell structure and function, biomolecules, and enzyme activity. It begins with an introduction to cell organelles, including the cell membrane, cytoplasm, mitochondria, endoplasmic reticulum, Golgi apparatus, lysosomes, and ribosomes, and discusses their respective roles. The report then delves into biomolecules, specifically carbohydrates, proteins, lipids, and nucleic acids, detailing their structures and functions within the cell. Furthermore, it explains the methods by which substances move in and out of cells, including passive and active transport mechanisms. The report also focuses on enzymes, discussing their classification, the factors affecting their activity (pH, concentration, and temperature), and the theories behind enzyme-substrate interactions. The report is a detailed analysis of cell structure, its components, and processes, offering a solid foundation in cell biology.

The structure of the cell
The structure of the cell.
(Name)
Centre Wiltshire College.
Cell Biology.
Barbara Stephens
(Date of submission)
The structure of the cell.
(Name)
Centre Wiltshire College.
Cell Biology.
Barbara Stephens
(Date of submission)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1The structure of the cell.
Table of contents
Cell structure and functions.............................................................................................................2
Biomolecules...................................................................................................................................3
Proteins.........................................................................................................................................4
Lipids...........................................................................................................................................5
Nucleic acids................................................................................................................................6
Methods that substances use to move in and out of cells................................................................7
Enzymes...........................................................................................................................................8
Introduction..................................................................................................................................8
Hypothesis....................................................................................................................................9
Methods........................................................................................................................................9
Results..........................................................................................................................................9
Discussion..................................................................................................................................10
Effects of PH on enzymes......................................................................................................10
Enzyme concentration............................................................................................................10
Substrate concentrations............................................................................................................11
Conclusion.....................................................................................................................................11
Table of contents
Cell structure and functions.............................................................................................................2
Biomolecules...................................................................................................................................3
Proteins.........................................................................................................................................4
Lipids...........................................................................................................................................5
Nucleic acids................................................................................................................................6
Methods that substances use to move in and out of cells................................................................7
Enzymes...........................................................................................................................................8
Introduction..................................................................................................................................8
Hypothesis....................................................................................................................................9
Methods........................................................................................................................................9
Results..........................................................................................................................................9
Discussion..................................................................................................................................10
Effects of PH on enzymes......................................................................................................10
Enzyme concentration............................................................................................................10
Substrate concentrations............................................................................................................11
Conclusion.....................................................................................................................................11

2The structure of the cell.
Cell structure and functions.
Figure 1: An image of cell organelles (Bobysheva & Kachshenko 2011).
The cell membrane is the outer part of the cell that has lipids, oligosaccharides and
proteins. The basic structure of the cell membrane consists of an arrangement of phospholipids
molecules each consisting of a polar hydrophilic head and a hydrophobic tail. In addition, there
are integral and peripheral proteins (Albert, Johnson& Lewis 2002). The cell membrane
maintains the structural integrity of the cell. Moreover, it allows movement of the cell through
formation of cilia and flagella. Finally, it regulates movement of substances in and out of the cell
(Cooper 2000).
The cytoplasm lies in between the nucleus and the cell membrane. It has a matrix that has
organelles and inclusions. Cell activities occur in the cytoplasm (Albert, Johnson& Lewis 2002).
Furthermore, the cytoplasm keeps organelles in place. The mitochondria has two membranes
divided by an inter membrane space. Folds of the inner membrane make the cristae, which
encloses the matrix space. Furthermore, it consists of ribosomes, t- RNA (Ribonucleic Acid) and
Cell structure and functions.
Figure 1: An image of cell organelles (Bobysheva & Kachshenko 2011).
The cell membrane is the outer part of the cell that has lipids, oligosaccharides and
proteins. The basic structure of the cell membrane consists of an arrangement of phospholipids
molecules each consisting of a polar hydrophilic head and a hydrophobic tail. In addition, there
are integral and peripheral proteins (Albert, Johnson& Lewis 2002). The cell membrane
maintains the structural integrity of the cell. Moreover, it allows movement of the cell through
formation of cilia and flagella. Finally, it regulates movement of substances in and out of the cell
(Cooper 2000).
The cytoplasm lies in between the nucleus and the cell membrane. It has a matrix that has
organelles and inclusions. Cell activities occur in the cytoplasm (Albert, Johnson& Lewis 2002).
Furthermore, the cytoplasm keeps organelles in place. The mitochondria has two membranes
divided by an inter membrane space. Folds of the inner membrane make the cristae, which
encloses the matrix space. Furthermore, it consists of ribosomes, t- RNA (Ribonucleic Acid) and
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3The structure of the cell.
mitochondrial DNA. The mitochondria produce chemical energy for the cell in form of ATP
(Adenosine Tri-phosphate) (Cooper 2000).
The endoplasmic reticulum appears as a rich network of flattened tubes and sacs bound
by a membrane. It is the site of carbohydrate, protein and lipid synthesis. The rough endoplasmic
reticulum is involved in protein synthesis, it is found in cells that synthesize proteins. It
synthesizes and segregates proteins for intracellular or extracellular use. The smooth
endoplasmic reticulum appears as membranous network in the cell. It is involved in synthesis of
steroids, detoxification and lipid metabolism (Bobrysheva & Kachshenko 2011).
The Golgi apparatus has a series of flattened, stacked membrane bound sacs, tubular
extensions and vesicles. The Golgi apparatus stores proteins from the endoplasmic reticulum for
secretion. Furthermore, it secretes lysosomes. Lysosomes are cell organelles involved in turnover
of cellular components and intracellular digestion. They are membrane bound spherical vesicles
that have a huge variety of hydrolytic enzymes. They are found in cells involved in phagocytic
activity such as macrophages (Bobrysheva & Kachshenko 2011).
Ribosomes are located in the rough endoplasmic reticulum or cytoplasm. They occur
singly (monosomes) or in groups (polysomes). Monosomes and polysomes secrete proteins used
in and out of the cell. The cell also consists of microfilaments, microtubules and intermediate
filaments, these are structural proteins that provide shape and form to the cell. They also play a
fundamental role in cellular and cytoplasmic movements. Microtubules are circular and they
provide a platform for cytoplasm components such as basal bodies, centrioles, cilia and flagella.
Centrioles are cylindrical in shape and lie at right angles with each other. Moreover, they are
mitochondrial DNA. The mitochondria produce chemical energy for the cell in form of ATP
(Adenosine Tri-phosphate) (Cooper 2000).
The endoplasmic reticulum appears as a rich network of flattened tubes and sacs bound
by a membrane. It is the site of carbohydrate, protein and lipid synthesis. The rough endoplasmic
reticulum is involved in protein synthesis, it is found in cells that synthesize proteins. It
synthesizes and segregates proteins for intracellular or extracellular use. The smooth
endoplasmic reticulum appears as membranous network in the cell. It is involved in synthesis of
steroids, detoxification and lipid metabolism (Bobrysheva & Kachshenko 2011).
The Golgi apparatus has a series of flattened, stacked membrane bound sacs, tubular
extensions and vesicles. The Golgi apparatus stores proteins from the endoplasmic reticulum for
secretion. Furthermore, it secretes lysosomes. Lysosomes are cell organelles involved in turnover
of cellular components and intracellular digestion. They are membrane bound spherical vesicles
that have a huge variety of hydrolytic enzymes. They are found in cells involved in phagocytic
activity such as macrophages (Bobrysheva & Kachshenko 2011).
Ribosomes are located in the rough endoplasmic reticulum or cytoplasm. They occur
singly (monosomes) or in groups (polysomes). Monosomes and polysomes secrete proteins used
in and out of the cell. The cell also consists of microfilaments, microtubules and intermediate
filaments, these are structural proteins that provide shape and form to the cell. They also play a
fundamental role in cellular and cytoplasmic movements. Microtubules are circular and they
provide a platform for cytoplasm components such as basal bodies, centrioles, cilia and flagella.
Centrioles are cylindrical in shape and lie at right angles with each other. Moreover, they are
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
4The structure of the cell.
involved in cell division since they form mitotic spindles. Cilia and flagella are motile bodies for
cell movement (National Cancer Institute 2017).
Bio-molecules.
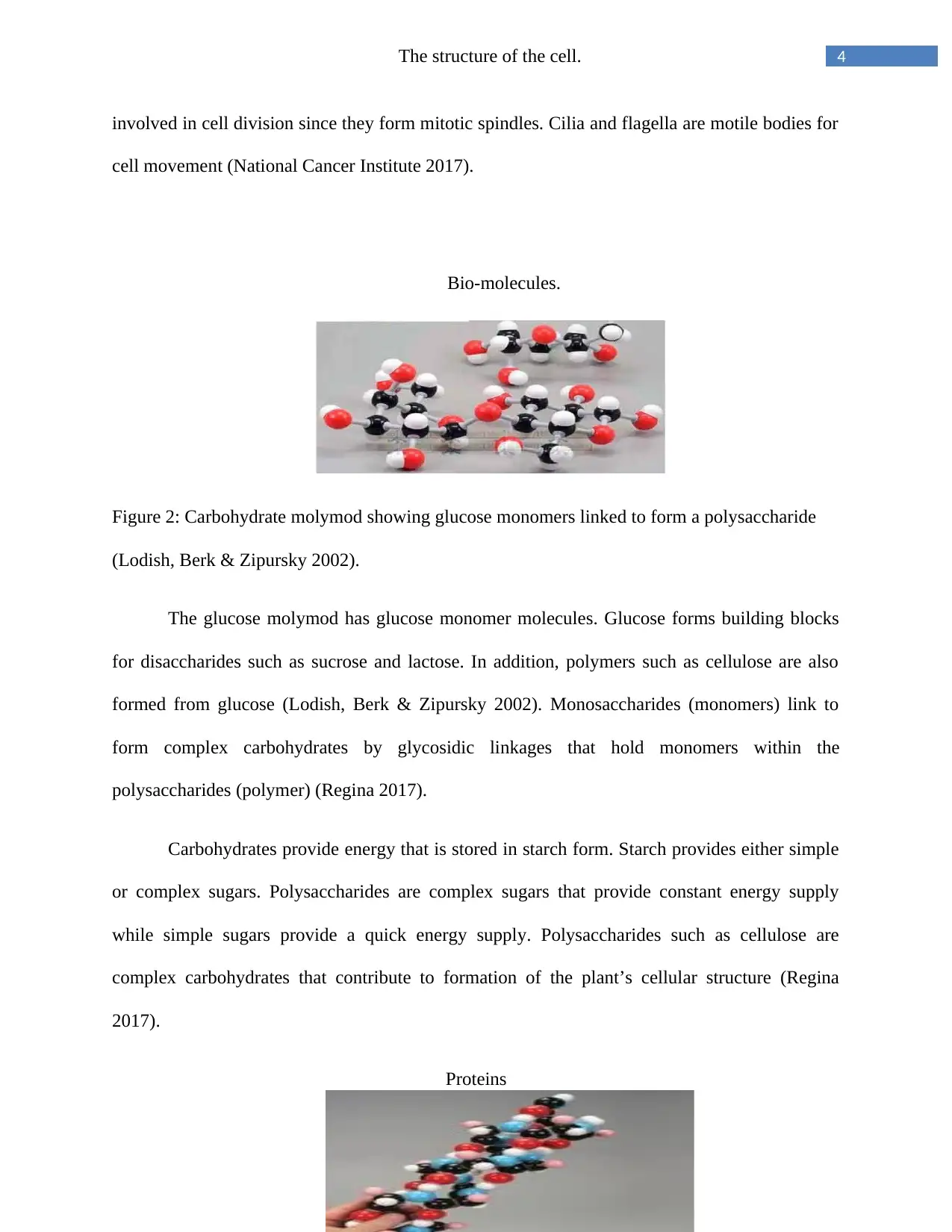
Figure 2: Carbohydrate molymod showing glucose monomers linked to form a polysaccharide
(Lodish, Berk & Zipursky 2002).
The glucose molymod has glucose monomer molecules. Glucose forms building blocks
for disaccharides such as sucrose and lactose. In addition, polymers such as cellulose are also
formed from glucose (Lodish, Berk & Zipursky 2002). Monosaccharides (monomers) link to
form complex carbohydrates by glycosidic linkages that hold monomers within the
polysaccharides (polymer) (Regina 2017).
Carbohydrates provide energy that is stored in starch form. Starch provides either simple
or complex sugars. Polysaccharides are complex sugars that provide constant energy supply
while simple sugars provide a quick energy supply. Polysaccharides such as cellulose are
complex carbohydrates that contribute to formation of the plant’s cellular structure (Regina
2017).
Proteins
involved in cell division since they form mitotic spindles. Cilia and flagella are motile bodies for
cell movement (National Cancer Institute 2017).
Bio-molecules.
Figure 2: Carbohydrate molymod showing glucose monomers linked to form a polysaccharide
(Lodish, Berk & Zipursky 2002).
The glucose molymod has glucose monomer molecules. Glucose forms building blocks
for disaccharides such as sucrose and lactose. In addition, polymers such as cellulose are also
formed from glucose (Lodish, Berk & Zipursky 2002). Monosaccharides (monomers) link to
form complex carbohydrates by glycosidic linkages that hold monomers within the
polysaccharides (polymer) (Regina 2017).
Carbohydrates provide energy that is stored in starch form. Starch provides either simple
or complex sugars. Polysaccharides are complex sugars that provide constant energy supply
while simple sugars provide a quick energy supply. Polysaccharides such as cellulose are
complex carbohydrates that contribute to formation of the plant’s cellular structure (Regina
2017).
Proteins

5The structure of the cell.
Figure 3: Protein molymod showing amino acids linked by peptide bonds (Stumpf & Conn
2004).
The protein alpha helix molymod structure is the most common secondary structure of
proteins. The structure has components that make up 15 peptide linkages. Proteins are made of
amino acid monomers that link via peptide bonds to form polypeptides (Stumpf & Conn 2004).
Proteins are the building blocks of the body, important in growth, repair and maintenance
of tissues. More proteins consumed in the body are used in energy production. Furthermore, they
are involved in formation of hormones, which regulate body functions. Enzymes are proteins that
act as catalysts for body chemical reactions. Proteins are also involved in the storage and
transport of molecules. Moreover, they form antibodies that fight diseases and infections (13).
Lipids
Figure 4: Lipid molymods (Regina 2017).
Lipid monomers are fatty acids that have a hydrocarbon chain attached to a carboxyl
group. Fatty acids form lipid polymers such as phospholipids, triglycerides and waxes. Lipids act
Figure 3: Protein molymod showing amino acids linked by peptide bonds (Stumpf & Conn
2004).
The protein alpha helix molymod structure is the most common secondary structure of
proteins. The structure has components that make up 15 peptide linkages. Proteins are made of
amino acid monomers that link via peptide bonds to form polypeptides (Stumpf & Conn 2004).
Proteins are the building blocks of the body, important in growth, repair and maintenance
of tissues. More proteins consumed in the body are used in energy production. Furthermore, they
are involved in formation of hormones, which regulate body functions. Enzymes are proteins that
act as catalysts for body chemical reactions. Proteins are also involved in the storage and
transport of molecules. Moreover, they form antibodies that fight diseases and infections (13).
Lipids
Figure 4: Lipid molymods (Regina 2017).
Lipid monomers are fatty acids that have a hydrocarbon chain attached to a carboxyl
group. Fatty acids form lipid polymers such as phospholipids, triglycerides and waxes. Lipids act
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
6The structure of the cell.
as chemical messengers that transfer information from one organelle to other cells through
chemical signaling. Furthermore, lipids store and provide energy. They are important in the
maintenance of body temperatures. The skin has a layer of subcutaneous fat, which insulates the
body and protects it from cold. Polyunsaturated fatty acids are important components of
phospholipids that provide several properties to the plasma membranes (Regina 2017).
Furthermore, lipids are involved in the formation of cholesterol. Cholesterol exists in
blood as plasma lipoproteins, a complex aggregate of proteins and lipids that enable movement
of lipids in the whole body. Cholesterol interacts with complex lipid components such as
phospholipids to maintain fluidity of membranes. Cholesterol is also a precursor of vitamin D,
bile acids and steroidal hormones (Donald & Judith 2003).
Nucleic acids
Figure 5: A DNA polymer consisting of nucleotide monomers (Stumpf & Conn 2004).
Nucleic acids monomers are nucleotides that link together to form polynucleotide chains
such as RNA and DNA. Furthermore, nucleic acids are genetic materials for living organisms.
They are involved in the transfer and storage of genetic material from one generation to another.
In addition, they determine the phenotype and characteristics of an organism. The DNA stores
as chemical messengers that transfer information from one organelle to other cells through
chemical signaling. Furthermore, lipids store and provide energy. They are important in the
maintenance of body temperatures. The skin has a layer of subcutaneous fat, which insulates the
body and protects it from cold. Polyunsaturated fatty acids are important components of
phospholipids that provide several properties to the plasma membranes (Regina 2017).
Furthermore, lipids are involved in the formation of cholesterol. Cholesterol exists in
blood as plasma lipoproteins, a complex aggregate of proteins and lipids that enable movement
of lipids in the whole body. Cholesterol interacts with complex lipid components such as
phospholipids to maintain fluidity of membranes. Cholesterol is also a precursor of vitamin D,
bile acids and steroidal hormones (Donald & Judith 2003).
Nucleic acids
Figure 5: A DNA polymer consisting of nucleotide monomers (Stumpf & Conn 2004).
Nucleic acids monomers are nucleotides that link together to form polynucleotide chains
such as RNA and DNA. Furthermore, nucleic acids are genetic materials for living organisms.
They are involved in the transfer and storage of genetic material from one generation to another.
In addition, they determine the phenotype and characteristics of an organism. The DNA stores
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
7The structure of the cell.
genetic information while the RNA transfers genetic information relayed from the nucleus to the
cytoplasm to initiate protein synthesis (Stumpf & Conn 2004).
Methods that substances use to move in and out of cells
Substances move in and out of cells by way of passive or active transport. Passive ways
include simple diffusion, facilitated diffusion and osmosis. The active ways of transport include:
pinocytosis, phagocytosis and sodium/potassium pump (Arthur, Guyton & John 2006).
A comparison of simple and facilitated diffusion.
Simple diffusion Facilitated diffusion
It does not require the assistance of carrier
molecules
Requires the assistance of a carrier molecule to
carry substances across the cells.
It has a relatively slow speed. The speed is comparatively fast.
It is not specific to solutes. It is always solute specific.
An inhibitor molecule cannot inhibit the
process of simple diffusion.
An inhibitor that binds specifically to the
carrier molecules can inhibit it.
The molecules involved in simple diffusion can
only pass in the direction of concentration
gradient.
The molecules can pass in both the opposite of
concentration gradient and in direction of the
concentration gradient.
They are both passive processes that do not require energy to move substances across the cell
membrane, movement takes place down the concentration gradient of molecules.
Active transport Passive transport.
It uses chemical energy, ATP, as a pump
to move molecules against a concentration
gradient.
It does not use ATP, nevertheless the molecules
move down the concentration gradient.
Movement occursfrom a low to a high
concentration of solutes.
Transport occurs from a highconcentration to a low
concentration of molecules.
genetic information while the RNA transfers genetic information relayed from the nucleus to the
cytoplasm to initiate protein synthesis (Stumpf & Conn 2004).
Methods that substances use to move in and out of cells
Substances move in and out of cells by way of passive or active transport. Passive ways
include simple diffusion, facilitated diffusion and osmosis. The active ways of transport include:
pinocytosis, phagocytosis and sodium/potassium pump (Arthur, Guyton & John 2006).
A comparison of simple and facilitated diffusion.
Simple diffusion Facilitated diffusion
It does not require the assistance of carrier
molecules
Requires the assistance of a carrier molecule to
carry substances across the cells.
It has a relatively slow speed. The speed is comparatively fast.
It is not specific to solutes. It is always solute specific.
An inhibitor molecule cannot inhibit the
process of simple diffusion.
An inhibitor that binds specifically to the
carrier molecules can inhibit it.
The molecules involved in simple diffusion can
only pass in the direction of concentration
gradient.
The molecules can pass in both the opposite of
concentration gradient and in direction of the
concentration gradient.
They are both passive processes that do not require energy to move substances across the cell
membrane, movement takes place down the concentration gradient of molecules.
Active transport Passive transport.
It uses chemical energy, ATP, as a pump
to move molecules against a concentration
gradient.
It does not use ATP, nevertheless the molecules
move down the concentration gradient.
Movement occursfrom a low to a high
concentration of solutes.
Transport occurs from a highconcentration to a low
concentration of molecules.

8The structure of the cell.
It needs cellular energy. It does not need cellular energy.
It transports proteins, large cells, complex
sugars and ions.
It transports soluble substances including
monosaccharides, lipids, water, carbon dioxide,
oxygen and sex hormones.
Table showing comparison of the way substances move in and out of the cell (Arthur, Guyton &
John 2006).
Enzymes
Introduction
According to Geetha et al. (2005, p. 47), enzymes are proteins that act as biological
catalysts. They support chemical reactions in the body that in turn maintain homeostasis. Two
theories define the mode in which enzymes act on substrates, these are the induced fit and the
lock and key theories. According to the lock and key theory proposed in 1894 by Emil Fisher, an
enzyme’s active site appears as a lock in which a substrate fits in like a key. Therefore, this
means that the active sites in enzymes and shapes of the substrate are complementary. The
enzyme and the substrate lock to form an enzyme substrate complex.
Daniel E. Koshland proposed the induced fit theory in 1959. It states that the active site in
an enzyme lacks a firm lock and key conformation. The shape of the active site is changed by the
binding of an enzyme to a substrate so that it complements the substrate molecules. Induced fit is
facilitated by flexibility of protein molecules (Worthington Biochemical Corporation 2006).The
two theories can be compared by analyzing their differences.
The lock and key hypothesis proposes that an enzyme’s active site is asingle entity while
the induced fit hypothesis proposes the active site to be divided into two components. Secondly,
the lock and key theory states that the catalytic group is not separate while the induced fit
hypothesis states that a separate catalytic group is evident. Finally, the lock and key hypothesis
It needs cellular energy. It does not need cellular energy.
It transports proteins, large cells, complex
sugars and ions.
It transports soluble substances including
monosaccharides, lipids, water, carbon dioxide,
oxygen and sex hormones.
Table showing comparison of the way substances move in and out of the cell (Arthur, Guyton &
John 2006).
Enzymes
Introduction
According to Geetha et al. (2005, p. 47), enzymes are proteins that act as biological
catalysts. They support chemical reactions in the body that in turn maintain homeostasis. Two
theories define the mode in which enzymes act on substrates, these are the induced fit and the
lock and key theories. According to the lock and key theory proposed in 1894 by Emil Fisher, an
enzyme’s active site appears as a lock in which a substrate fits in like a key. Therefore, this
means that the active sites in enzymes and shapes of the substrate are complementary. The
enzyme and the substrate lock to form an enzyme substrate complex.
Daniel E. Koshland proposed the induced fit theory in 1959. It states that the active site in
an enzyme lacks a firm lock and key conformation. The shape of the active site is changed by the
binding of an enzyme to a substrate so that it complements the substrate molecules. Induced fit is
facilitated by flexibility of protein molecules (Worthington Biochemical Corporation 2006).The
two theories can be compared by analyzing their differences.
The lock and key hypothesis proposes that an enzyme’s active site is asingle entity while
the induced fit hypothesis proposes the active site to be divided into two components. Secondly,
the lock and key theory states that the catalytic group is not separate while the induced fit
hypothesis states that a separate catalytic group is evident. Finally, the lock and key hypothesis
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
9The structure of the cell.
states that it does not consider the development of a transition state while the induced fit theory
considers development of transition state before there is change in reactant (Worthington
Biochemical Corporation 2006).
Enzymes are classified according to the reactions they catalyse. Some enzymes catalyse
the removal or addition of water into chemical reactions. For example, carbohydrases, esterases,
deaminases and nucleases. Others such as dehydrogenases and oxidases,catalyse transfer of
electrons. In addition, some enzymes catalyse the transfer of radicals (Theresa 2017).
Hypothesis.
Enzyme concentration, substrate concentration, temperature, and pH affect the activity of
enzymes in various ways.
Methods.
The research study collected and analyzed data on the pH, concentration and temperature
values against the rate of reaction of enzymes. The association between the factors and enzymes
was noted and the information was presented in graphs to indicate how the factors affect the
enzymes.
Results.
states that it does not consider the development of a transition state while the induced fit theory
considers development of transition state before there is change in reactant (Worthington
Biochemical Corporation 2006).
Enzymes are classified according to the reactions they catalyse. Some enzymes catalyse
the removal or addition of water into chemical reactions. For example, carbohydrases, esterases,
deaminases and nucleases. Others such as dehydrogenases and oxidases,catalyse transfer of
electrons. In addition, some enzymes catalyse the transfer of radicals (Theresa 2017).
Hypothesis.
Enzyme concentration, substrate concentration, temperature, and pH affect the activity of
enzymes in various ways.
Methods.
The research study collected and analyzed data on the pH, concentration and temperature
values against the rate of reaction of enzymes. The association between the factors and enzymes
was noted and the information was presented in graphs to indicate how the factors affect the
enzymes.
Results.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
10The structure of the cell.
Discussion.
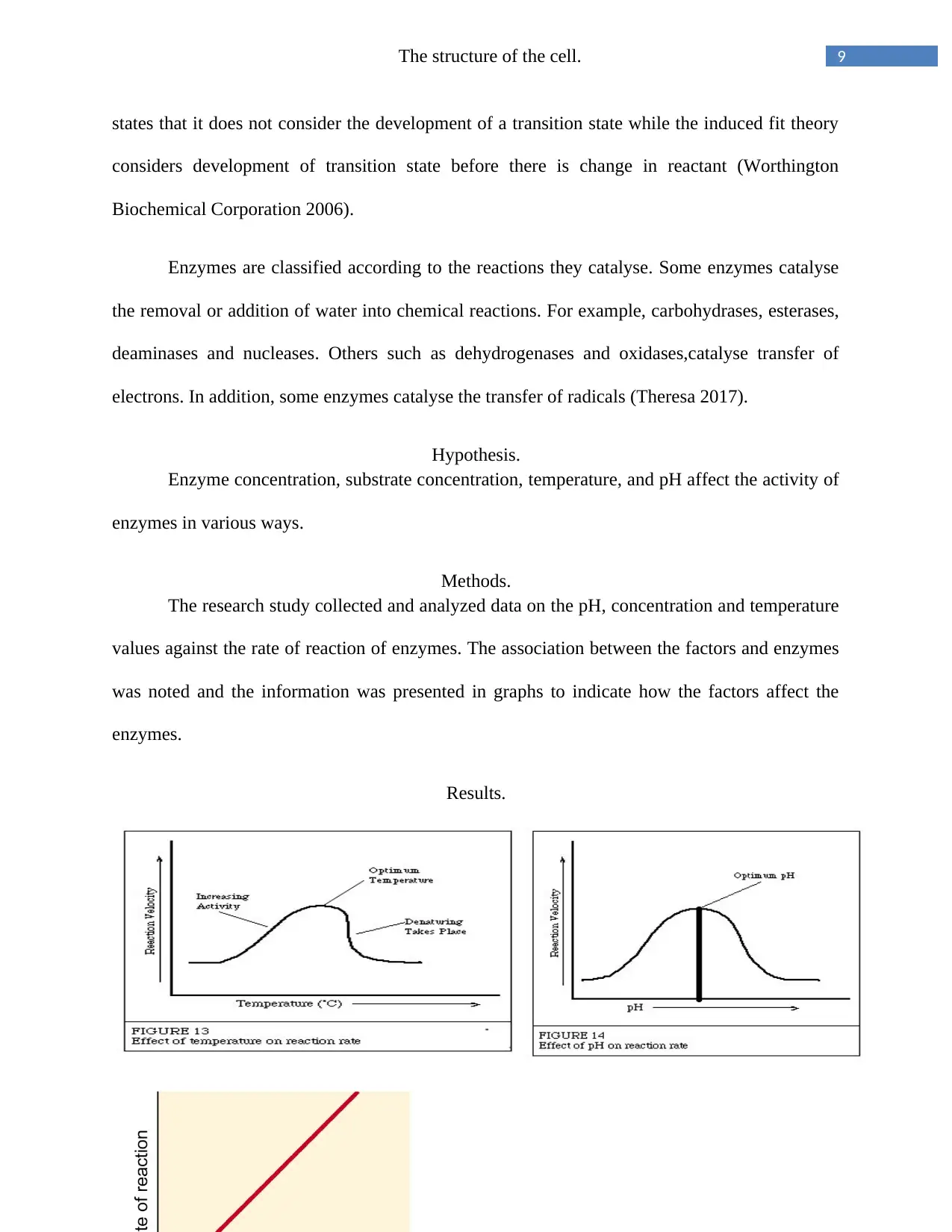
The rate of reaction of an enzyme increases with an increase in temperature. For instance,
when the temperature of an enzyme is increased, the activity of most enzymes also
increases.High temperatures affect the rate of reaction of many enzymes as shown in the figure
above. The rate of reaction increases to an upper limit level then a further increase in temperature
abruptly lowers the rate of reaction. Enzymes are rapidly denatured by temperatures higher than
400C. After a given time, they are deactivated at moderate temperatures (Theresa 2017).
Effects of PH on enzymes.
All enzymes work best at their own optimal pH. The optimal pH of an enzyme varies
from one enzyme to another.The pH of an enzyme affects its stability, very high or low PHs
result in a complete loss of activity. For instance, lipase an enzyme in the pancreas has an
optimum pH of 8.0, pepsin has an enzyme range of 1. 5 to 1.6 (Donald & Judith 2003) . The
enzymes cannot function if its pH is far from the enzyme’s optimal pH. Reduction in enzyme
efficiency due to change in pH is as a result of change in degree of ionization of the enzyme and
the substrate .Extremely acidic or alkaline conditions denature enzymes (Theresa 2017).
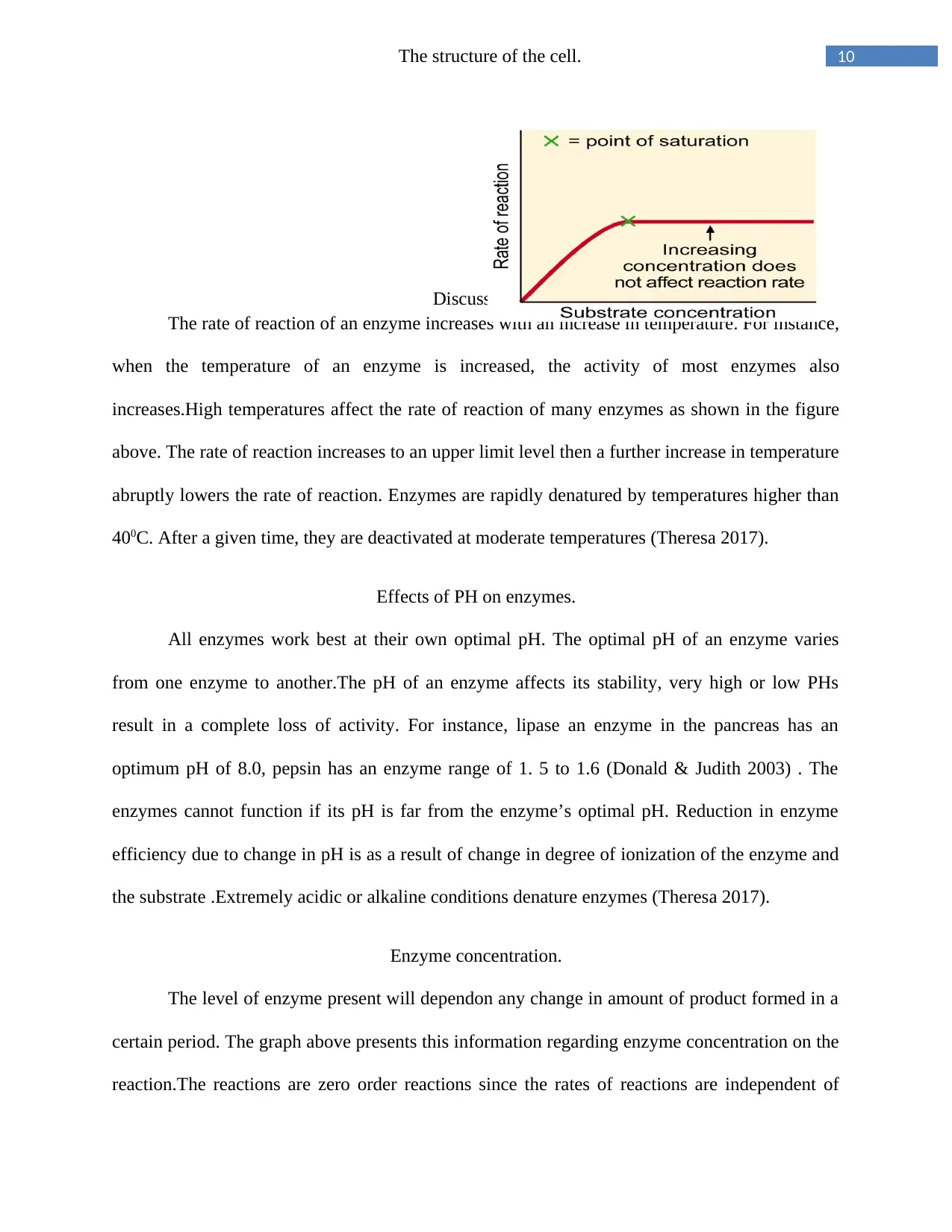
Enzyme concentration.
The level of enzyme present will dependon any change in amount of product formed in a
certain period. The graph above presents this information regarding enzyme concentration on the
reaction.The reactions are zero order reactions since the rates of reactions are independent of
Discussion.
The rate of reaction of an enzyme increases with an increase in temperature. For instance,
when the temperature of an enzyme is increased, the activity of most enzymes also
increases.High temperatures affect the rate of reaction of many enzymes as shown in the figure
above. The rate of reaction increases to an upper limit level then a further increase in temperature
abruptly lowers the rate of reaction. Enzymes are rapidly denatured by temperatures higher than
400C. After a given time, they are deactivated at moderate temperatures (Theresa 2017).
Effects of PH on enzymes.
All enzymes work best at their own optimal pH. The optimal pH of an enzyme varies
from one enzyme to another.The pH of an enzyme affects its stability, very high or low PHs
result in a complete loss of activity. For instance, lipase an enzyme in the pancreas has an
optimum pH of 8.0, pepsin has an enzyme range of 1. 5 to 1.6 (Donald & Judith 2003) . The
enzymes cannot function if its pH is far from the enzyme’s optimal pH. Reduction in enzyme
efficiency due to change in pH is as a result of change in degree of ionization of the enzyme and
the substrate .Extremely acidic or alkaline conditions denature enzymes (Theresa 2017).
Enzyme concentration.
The level of enzyme present will dependon any change in amount of product formed in a
certain period. The graph above presents this information regarding enzyme concentration on the
reaction.The reactions are zero order reactions since the rates of reactions are independent of

11The structure of the cell.
substrate concentrations. Product formation occurs at a rate of reaction linear with time. The
amount of enzyme present in a reaction quantifies the activity catalyzed. In the figure above,
theenzyme activity is greatest when the enzyme concentration is in excess (Theresa 2017).
Substrate concentration
Experiments have shown that when enzyme concentration is constant, an increase in
substrate concentration leads to an increase in the rate of reaction to a maximum. After the
maximum, the rate of reaction will not increase even with an increase in the substrate
concentration. It is presented graphically in figure above. When the maximum velocity of an
enzyme reaction has been reached, all the enzyme available is converted to a product known as
the enzyme substrate complex. This point is the point of saturation on the graph (Nelson et al
1999).
substrate concentrations. Product formation occurs at a rate of reaction linear with time. The
amount of enzyme present in a reaction quantifies the activity catalyzed. In the figure above,
theenzyme activity is greatest when the enzyme concentration is in excess (Theresa 2017).
Substrate concentration
Experiments have shown that when enzyme concentration is constant, an increase in
substrate concentration leads to an increase in the rate of reaction to a maximum. After the
maximum, the rate of reaction will not increase even with an increase in the substrate
concentration. It is presented graphically in figure above. When the maximum velocity of an
enzyme reaction has been reached, all the enzyme available is converted to a product known as
the enzyme substrate complex. This point is the point of saturation on the graph (Nelson et al
1999).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 14
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





