The Structure Periodic Table and Bonding
Calculate the relative atomic mass of given elements and explain the calculations.
11 Pages1560 Words22 Views
Added on 2022-09-07
The Structure Periodic Table and Bonding
Calculate the relative atomic mass of given elements and explain the calculations.
Added on 2022-09-07
ShareRelated Documents
The Structure, Periodic Table, and Bonding
PRIYANTHI THILLAINATHAN (40142414) 1
Task 1 (AC 1.2, 1.3 and 4.1)
Part 1 (AC 1.2)
You will compute the relative atomic mass of your given elements from the data provided in
Information Sheets 1, 2 and 3 and write a paragraph to explain your calculations.
Atomic structure
An atom has a central nucleus. The nucleus has neutrons (0 charges) and positive charge protons. It is
covered by negative charge electrons organized in shells. It has no general charge; it is neutral.
Number of neutrons + Number of protons =Mass number
Number of protons = Atomic number
Number of electrons = Number of protons
The relative atomic mass (Ar) is the ratio of its atoms mass compared to 1
12 of the mass of carbon-12
atom.
This weight depends on two factors:
1. The number of protons & neutrons in its isotopes.
2. Quantity of these isotopes.
Isotopes are atoms of a given element with an exact quantity of electrons and protons with a different
number of neutrons. The atomic number is the same, but mass numbers are different (BBC Bitesize.
2019).
PRIYANTHI THILLAINATHAN (40142414) 1
Task 1 (AC 1.2, 1.3 and 4.1)
Part 1 (AC 1.2)
You will compute the relative atomic mass of your given elements from the data provided in
Information Sheets 1, 2 and 3 and write a paragraph to explain your calculations.
Atomic structure
An atom has a central nucleus. The nucleus has neutrons (0 charges) and positive charge protons. It is
covered by negative charge electrons organized in shells. It has no general charge; it is neutral.
Number of neutrons + Number of protons =Mass number
Number of protons = Atomic number
Number of electrons = Number of protons
The relative atomic mass (Ar) is the ratio of its atoms mass compared to 1
12 of the mass of carbon-12
atom.
This weight depends on two factors:
1. The number of protons & neutrons in its isotopes.
2. Quantity of these isotopes.
Isotopes are atoms of a given element with an exact quantity of electrons and protons with a different
number of neutrons. The atomic number is the same, but mass numbers are different (BBC Bitesize.
2019).

The Structure, Periodic Table, and Bonding
PRIYANTHI THILLAINATHAN (40142414) 2
From sheet 3, allocated elements are magnesium and chromium.
Relative atomic masses of the element from sheet 1.
The relative atomic mass of Magnesium.
Magnesium isotopes Percentage
26 Mg 11.01
25 Mg 10.00
24 Mg 78.99
Ar = (mass of isotopes 1 x % of isotopes 1) + (mass of isotopes 2 x % of isotopes 2)
100
Ar of magnesium = (24 x 78.99) + (25 x 10) + (26 x 11.01)
100
= 24.32
The relative atomic mass of chromium
Chromium isotopes Percentage abundance
54 Cr 2.365
53 Cr 9.501
52 Cr 83.789
50 Cr 4.345
Ar of chromium = (50 x 4.345) + (52 x 83.789) + (53 x 9.501) + (54 x 2.365)
100
= 52.06
PRIYANTHI THILLAINATHAN (40142414) 2
From sheet 3, allocated elements are magnesium and chromium.
Relative atomic masses of the element from sheet 1.
The relative atomic mass of Magnesium.
Magnesium isotopes Percentage
26 Mg 11.01
25 Mg 10.00
24 Mg 78.99
Ar = (mass of isotopes 1 x % of isotopes 1) + (mass of isotopes 2 x % of isotopes 2)
100
Ar of magnesium = (24 x 78.99) + (25 x 10) + (26 x 11.01)
100
= 24.32
The relative atomic mass of chromium
Chromium isotopes Percentage abundance
54 Cr 2.365
53 Cr 9.501
52 Cr 83.789
50 Cr 4.345
Ar of chromium = (50 x 4.345) + (52 x 83.789) + (53 x 9.501) + (54 x 2.365)
100
= 52.06
The Structure, Periodic Table, and Bonding
PRIYANTHI THILLAINATHAN (40142414) 3
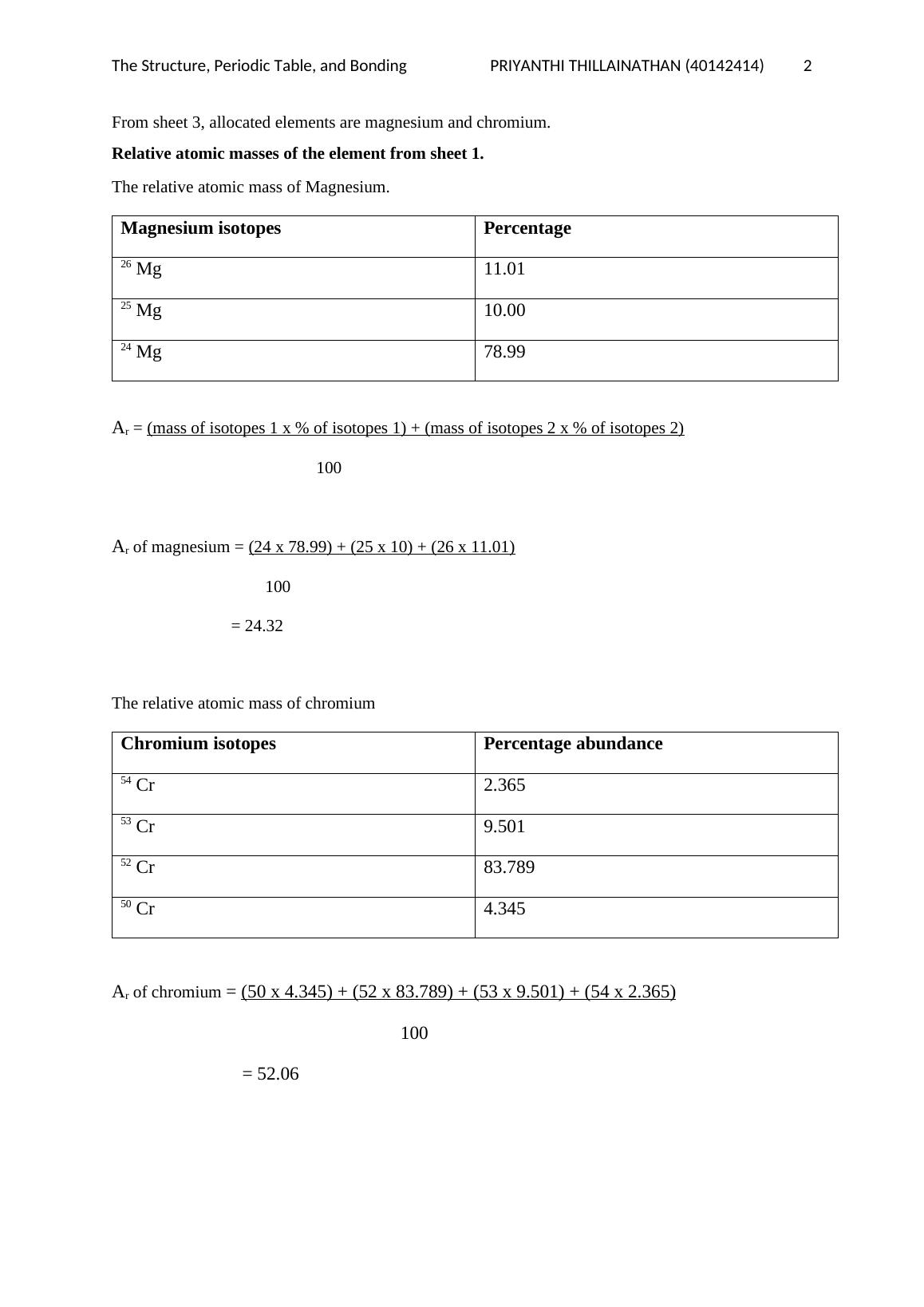
Relative Atomic Masses of elements provided in sheet 2.
Ar of magnesium = (24 x 78.70) + (26 x 10.13) + (26 x 11.17)
100
= 24.43
Relative atomic mass of Chromium
Ar of chromium = (50 x 5.0) + (52 x 85.00) + (53 x 10.0)
100
= 52.00
PRIYANTHI THILLAINATHAN (40142414) 3
Relative Atomic Masses of elements provided in sheet 2.
Ar of magnesium = (24 x 78.70) + (26 x 10.13) + (26 x 11.17)
100
= 24.43
Relative atomic mass of Chromium
Ar of chromium = (50 x 5.0) + (52 x 85.00) + (53 x 10.0)
100
= 52.00
The Structure, Periodic Table, and Bonding
PRIYANTHI THILLAINATHAN (40142414) 4
Explanation
The following steps are used to compute the atomic weight of a particular element (Anon, 2016).
Step 1: Multiply each isotope mass by its natural abundance; it the values are in percentage, divide the
answer by 100.
Step 2: Sum up the obtained values together
Either way;
Step 1: You can multiply each isotope by its percentage abundance and add them up.
Step 2: Then, divide the answer to the obtained answer by 100.
Part 2 (AC 1.3)
You will use the information provided in Information Sheet 3 to deduce the electronic
configurations, in terms of subshells, of your two allocated atoms and ions.
The electronic configuration is defined by how the electrons are organized in the energy levels
surrounding the atom nucleus.
Atomic orbitals are situated within electron shells. Within an electron shell, some subshells consist of
one or more orbitals. An orbital may have a limit of 2 electrons with inverse turn.
An electron occupies any 1 of the four types of orbital called f, d, p, and s orbitals.
Subshells orbitals number Highest electrons number
F 7 14
D 5 10
PRIYANTHI THILLAINATHAN (40142414) 4
Explanation
The following steps are used to compute the atomic weight of a particular element (Anon, 2016).
Step 1: Multiply each isotope mass by its natural abundance; it the values are in percentage, divide the
answer by 100.
Step 2: Sum up the obtained values together
Either way;
Step 1: You can multiply each isotope by its percentage abundance and add them up.
Step 2: Then, divide the answer to the obtained answer by 100.
Part 2 (AC 1.3)
You will use the information provided in Information Sheet 3 to deduce the electronic
configurations, in terms of subshells, of your two allocated atoms and ions.
The electronic configuration is defined by how the electrons are organized in the energy levels
surrounding the atom nucleus.
Atomic orbitals are situated within electron shells. Within an electron shell, some subshells consist of
one or more orbitals. An orbital may have a limit of 2 electrons with inverse turn.
An electron occupies any 1 of the four types of orbital called f, d, p, and s orbitals.
Subshells orbitals number Highest electrons number
F 7 14
D 5 10

End of preview
Want to access all the pages? Upload your documents or become a member.
Related Documents
o .. NUCLEI. Atomic mass unit (u) An atomic mass unit ilg...
|10
|3687
|2
Chemistry Question Answer 2022lg...
|9
|1649
|32
