Thermochemical Equation for Combustion of Octane and Bond Enthalpy
VerifiedAdded on 2023/04/23
|6
|1517
|352
AI Summary
This document explains the thermochemical equation for the combustion of octane, bond enthalpy, and an experiment on the reaction between ethanoic acid and calcium carbonate. It also covers the difference between bond dissociation enthalpy and standard mean bond enthalpy.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Chemistry
1. The thermochemical equation for a complete combustion of octane is given as:
2 C8 H18 ( l ) +25 O2 ( g ) → 16 C O2 ( g ) +18 H2 O ( l ) ∆ H °=−10 860 Kj mol−1
a) Exothermic and endothermic reaction
i) An exothermic reaction are reactions that releases heat, causing the temperature
of the surroundings to rise, thus one feel worm. An endothermic reactions are
reactions that absorbs heat and cools the surroundings, thus one feel cold.
ii) From the above reaction, the reaction is exothermic reaction since the enthalpy
change is negative, hence the temperature is lost to the surrounding
b) Standard enthalpy of combustion of a substance is the change in enthalpy when one mole
of a substance in the standard state (25 °C=298.15°K of temperature and 1 atmosphere
=101.325kpa of pressure) is formed from its pure elements under the same conditions.
c) The enthalpy change of formation of the above compounds are as:
8 C ( s ) +9 H2 ( g ) →C8 H18 ( l ) ∆ H °f =−249.73 kJ mol−1
C ( s ) +O2 ( g ) → C O2 ( g ) ∆ H °f =−−393.51kJ mol−1
2 H2 ( g ) +O2 ( g ) → 2 H2 O ( l ) ∆ H °f =−−−285.83 kJ mol−1
At standard pressure and temperature, the enthalpy change of formation of oxygen is
0.Now using Hess law, the enthalpy change of combustion of octane is:
∆ H °=16 (−393.51 ) + 18
2 (−285.83 )−2 (−249.73 )¿−8369.17 kJ mol−1
d) The heat (energy) released when a complete combustion of 2.25 moles of octane under
STP
Since 2 mol of octane releases 10860 kj, then 2.25 mol will release
1. The thermochemical equation for a complete combustion of octane is given as:
2 C8 H18 ( l ) +25 O2 ( g ) → 16 C O2 ( g ) +18 H2 O ( l ) ∆ H °=−10 860 Kj mol−1
a) Exothermic and endothermic reaction
i) An exothermic reaction are reactions that releases heat, causing the temperature
of the surroundings to rise, thus one feel worm. An endothermic reactions are
reactions that absorbs heat and cools the surroundings, thus one feel cold.
ii) From the above reaction, the reaction is exothermic reaction since the enthalpy
change is negative, hence the temperature is lost to the surrounding
b) Standard enthalpy of combustion of a substance is the change in enthalpy when one mole
of a substance in the standard state (25 °C=298.15°K of temperature and 1 atmosphere
=101.325kpa of pressure) is formed from its pure elements under the same conditions.
c) The enthalpy change of formation of the above compounds are as:
8 C ( s ) +9 H2 ( g ) →C8 H18 ( l ) ∆ H °f =−249.73 kJ mol−1
C ( s ) +O2 ( g ) → C O2 ( g ) ∆ H °f =−−393.51kJ mol−1
2 H2 ( g ) +O2 ( g ) → 2 H2 O ( l ) ∆ H °f =−−−285.83 kJ mol−1
At standard pressure and temperature, the enthalpy change of formation of oxygen is
0.Now using Hess law, the enthalpy change of combustion of octane is:
∆ H °=16 (−393.51 ) + 18
2 (−285.83 )−2 (−249.73 )¿−8369.17 kJ mol−1
d) The heat (energy) released when a complete combustion of 2.25 moles of octane under
STP
Since 2 mol of octane releases 10860 kj, then 2.25 mol will release
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

∆ H °=−10 860 Kj mol−1
2 mol ×2.25 mol=−12217.5 kJ
2.25 moles of octane releases 12217.5 kJ of heat
e) If 0.500 g of octane is completely heated, the energy(heat) releases is:
moles of C8 H18= mass
molar mass = 0.500
114.26 =0.00437598mol
∆ H °=−10 860 Kj mol−1
2 mol ×0.00437598 mol=−23.76159636 kJ
Assuming all heat released by combustion of gas was fully absorbed by 500 cm3 of water,
then the water rises by:
mwater =Vρ=500 g × 1.00 gc m−3=500 g∨0.50 kg Q=cm ∆T
∴ ∆ T = Q
cm = 237615.9636 J
4.18 J K−1 g−1 ×500 g =11.3692°
f) Incomplete combustion of octane
i) The equation is given below
2 C8 H18 ( l ) +9 O2 ( g ) → 16 C ( s ) +18 H2 O ( l )
ii) Under standard conditions, one mole of incomplete combustion of octane will
produce less energy as compared to one mole of complete combustion of octane.
This is because in incomplete combustion, no enthalpy change of formation of
carbon (IV) oxide. The enthalpy change of incomplete combustion of 1 mole will
be:
∆ H °= 1
2 (18
2 (−285.83 )−2 (−249.73 ) )=−1036.51 kJ mol−1
And the energy released by one mole of complete combustion is:
−8369.17 × 1
2 =−4184.59 kJ mo l−1
2 mol ×2.25 mol=−12217.5 kJ
2.25 moles of octane releases 12217.5 kJ of heat
e) If 0.500 g of octane is completely heated, the energy(heat) releases is:
moles of C8 H18= mass
molar mass = 0.500
114.26 =0.00437598mol
∆ H °=−10 860 Kj mol−1
2 mol ×0.00437598 mol=−23.76159636 kJ
Assuming all heat released by combustion of gas was fully absorbed by 500 cm3 of water,
then the water rises by:
mwater =Vρ=500 g × 1.00 gc m−3=500 g∨0.50 kg Q=cm ∆T
∴ ∆ T = Q
cm = 237615.9636 J
4.18 J K−1 g−1 ×500 g =11.3692°
f) Incomplete combustion of octane
i) The equation is given below
2 C8 H18 ( l ) +9 O2 ( g ) → 16 C ( s ) +18 H2 O ( l )
ii) Under standard conditions, one mole of incomplete combustion of octane will
produce less energy as compared to one mole of complete combustion of octane.
This is because in incomplete combustion, no enthalpy change of formation of
carbon (IV) oxide. The enthalpy change of incomplete combustion of 1 mole will
be:
∆ H °= 1
2 (18
2 (−285.83 )−2 (−249.73 ) )=−1036.51 kJ mol−1
And the energy released by one mole of complete combustion is:
−8369.17 × 1
2 =−4184.59 kJ mo l−1

iii) The reason for released energy to be less is because, the enthalpy change of
formation of CO2 is not in the incomplete combustion of octane
2. Bond enthalpy
a) Standard bond dissociation enthalpy of a bond is the amount of energy needed to
homolytically fracture one mole of a chemical bond.
b) Difference between bond dissociation enthalpy and standard mean bond enthalpy is that
the bond dissociation enthalpy refers to a specific bond within a specific molecule while
Mean bond enthalpy is the average for a particular type of bond measured over many
different types of molecule.
c) Mean bond enthalpy will:
i) decrease when bond strength is decreases since the attraction energy is decreased
ii) increase when bond length decreases, since the shorter the bond length, the
stronger the pull between the two atoms thus the higher the mean bond enthalpy
d) determining the standard enthalpy change of reaction of hydrogenation of but-1-ene
C H3 C H2 CH =C H2 ( g ) + H2 ( g) →C H3 C H2 CH2 C H3 ( g )
Bond breaking:
1 mol C=C=612 kJ mo l−12 mol C−C=2 ( 348 )=696 kJ mol−11 mol H−H =436 kJ mol−1
8 mol C−H =8 ( 413 ) =3304 kJ mol−1Total energy put∈¿ 5048 kJ mo l−1
Bond making
10 mol C−H=10 ( 413 )=4130 kJ mo l−13 mol C−C=3 ( 348 )=1044 kJ mo l−1
Total energy given out=5174 kJ mol−1∴ ∆ H=5048−5174=128 kJ mo l−1
e) Hydrogenation of but-1-ene using thermo-cycle, is determined as follows:
formation of CO2 is not in the incomplete combustion of octane
2. Bond enthalpy
a) Standard bond dissociation enthalpy of a bond is the amount of energy needed to
homolytically fracture one mole of a chemical bond.
b) Difference between bond dissociation enthalpy and standard mean bond enthalpy is that
the bond dissociation enthalpy refers to a specific bond within a specific molecule while
Mean bond enthalpy is the average for a particular type of bond measured over many
different types of molecule.
c) Mean bond enthalpy will:
i) decrease when bond strength is decreases since the attraction energy is decreased
ii) increase when bond length decreases, since the shorter the bond length, the
stronger the pull between the two atoms thus the higher the mean bond enthalpy
d) determining the standard enthalpy change of reaction of hydrogenation of but-1-ene
C H3 C H2 CH =C H2 ( g ) + H2 ( g) →C H3 C H2 CH2 C H3 ( g )
Bond breaking:
1 mol C=C=612 kJ mo l−12 mol C−C=2 ( 348 )=696 kJ mol−11 mol H−H =436 kJ mol−1
8 mol C−H =8 ( 413 ) =3304 kJ mol−1Total energy put∈¿ 5048 kJ mo l−1
Bond making
10 mol C−H=10 ( 413 )=4130 kJ mo l−13 mol C−C=3 ( 348 )=1044 kJ mo l−1
Total energy given out=5174 kJ mol−1∴ ∆ H=5048−5174=128 kJ mo l−1
e) Hydrogenation of but-1-ene using thermo-cycle, is determined as follows:
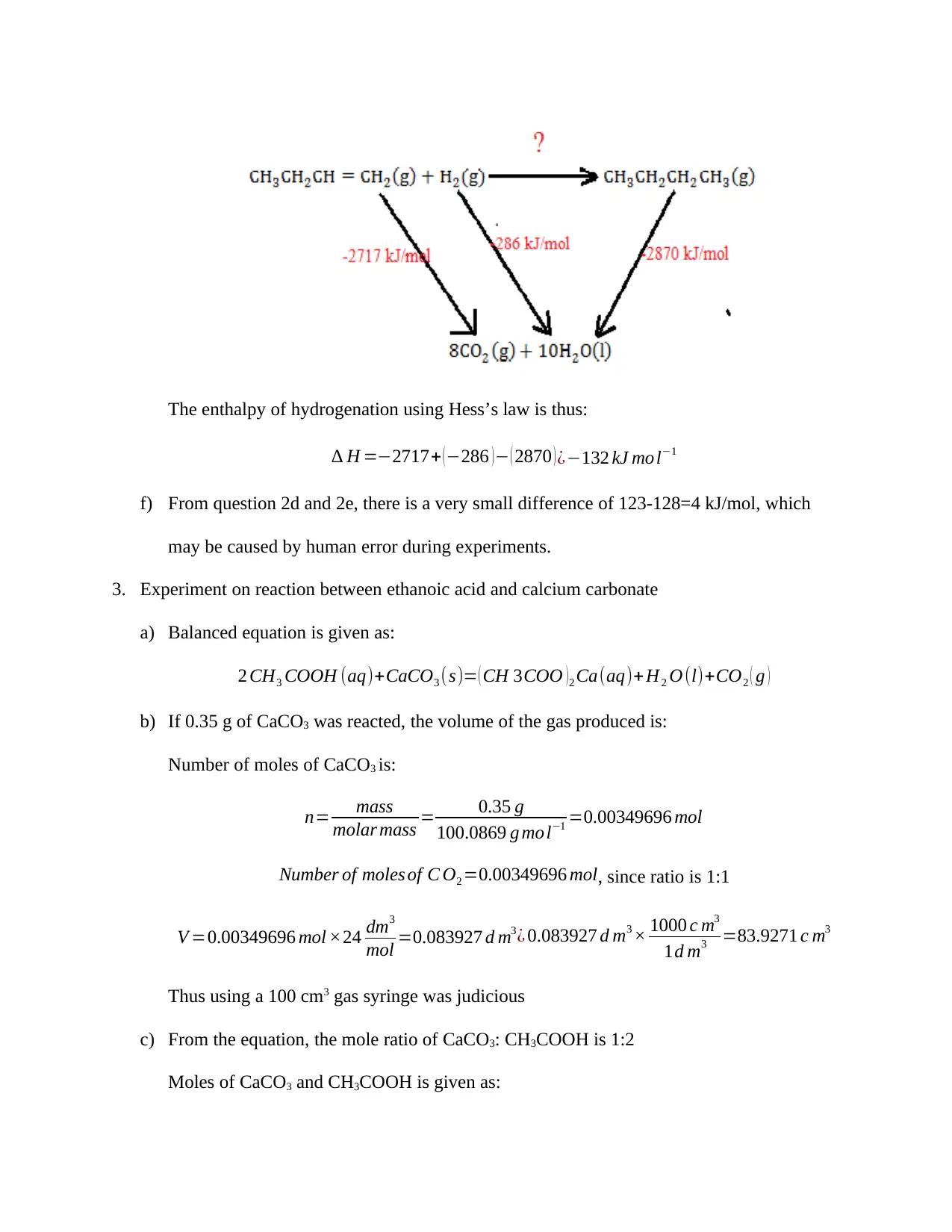
The enthalpy of hydrogenation using Hess’s law is thus:
∆ H =−2717+ ( −286 ) − ( 2870 ) ¿−132 kJ mo l−1
f) From question 2d and 2e, there is a very small difference of 123-128=4 kJ/mol, which
may be caused by human error during experiments.
3. Experiment on reaction between ethanoic acid and calcium carbonate
a) Balanced equation is given as:
2 CH3 COOH (aq)+CaCO3 (s)= ( CH 3COO ) 2 Ca(aq)+H2 O(l)+CO2 ( g )
b) If 0.35 g of CaCO3 was reacted, the volume of the gas produced is:
Number of moles of CaCO3 is:
n= mass
molar mass = 0.35 g
100.0869 g mo l−1 =0.00349696 mol
Number of moles of C O2 =0.00349696 mol, since ratio is 1:1
V =0.00349696 mol ×24 dm3
mol =0.083927 d m3¿ 0.083927 d m3 × 1000 c m3
1d m3 =83.9271 c m3
Thus using a 100 cm3 gas syringe was judicious
c) From the equation, the mole ratio of CaCO3: CH3COOH is 1:2
Moles of CaCO3 and CH3COOH is given as:
∆ H =−2717+ ( −286 ) − ( 2870 ) ¿−132 kJ mo l−1
f) From question 2d and 2e, there is a very small difference of 123-128=4 kJ/mol, which
may be caused by human error during experiments.
3. Experiment on reaction between ethanoic acid and calcium carbonate
a) Balanced equation is given as:
2 CH3 COOH (aq)+CaCO3 (s)= ( CH 3COO ) 2 Ca(aq)+H2 O(l)+CO2 ( g )
b) If 0.35 g of CaCO3 was reacted, the volume of the gas produced is:
Number of moles of CaCO3 is:
n= mass
molar mass = 0.35 g
100.0869 g mo l−1 =0.00349696 mol
Number of moles of C O2 =0.00349696 mol, since ratio is 1:1
V =0.00349696 mol ×24 dm3
mol =0.083927 d m3¿ 0.083927 d m3 × 1000 c m3
1d m3 =83.9271 c m3
Thus using a 100 cm3 gas syringe was judicious
c) From the equation, the mole ratio of CaCO3: CH3COOH is 1:2
Moles of CaCO3 and CH3COOH is given as:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
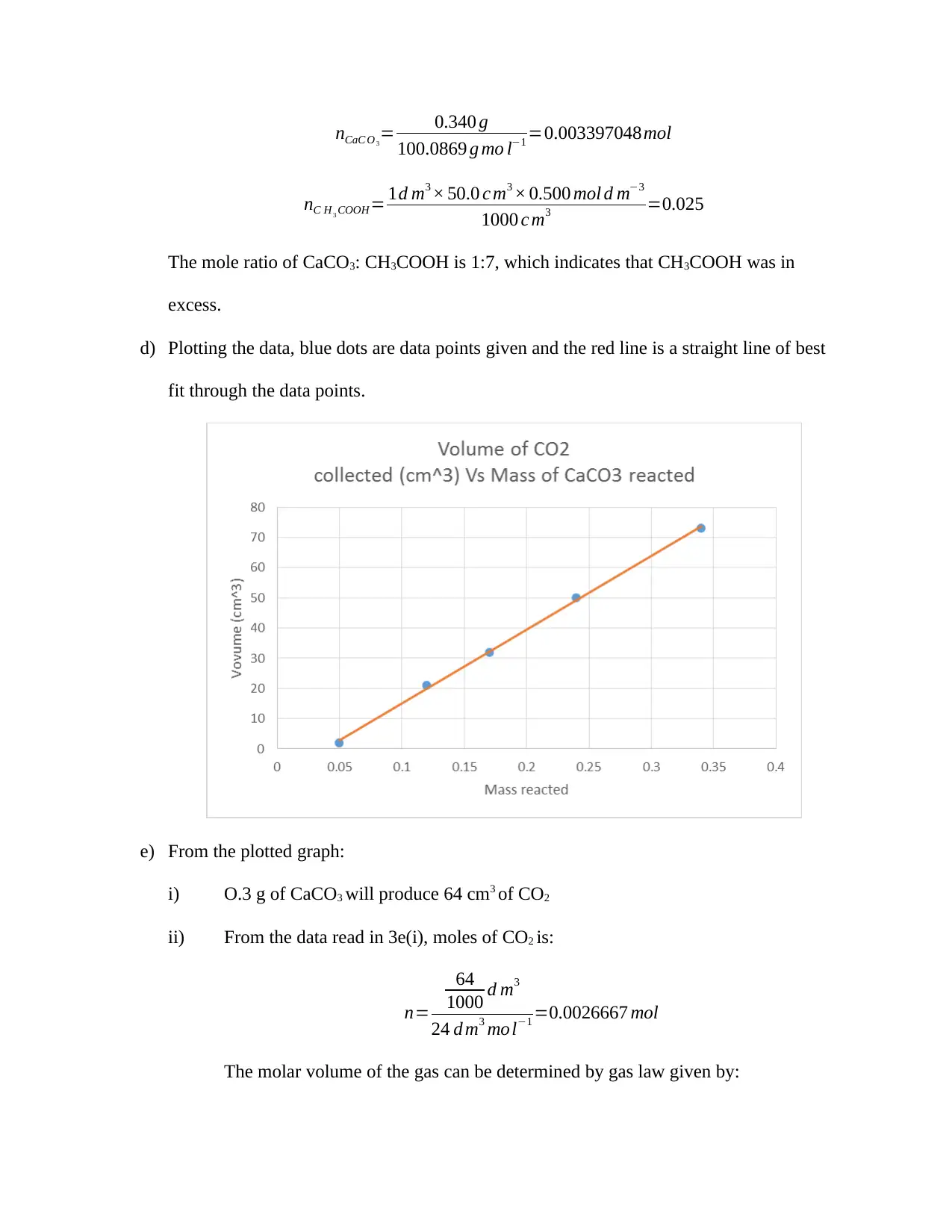
nCaC O 3
= 0.340 g
100.0869 g mo l−1 =0.003397048mol
nC H 3 COOH= 1d m3 × 50.0 c m3 × 0.500 mol d m−3
1000 c m3 =0.025
The mole ratio of CaCO3: CH3COOH is 1:7, which indicates that CH3COOH was in
excess.
d) Plotting the data, blue dots are data points given and the red line is a straight line of best
fit through the data points.
e) From the plotted graph:
i) O.3 g of CaCO3 will produce 64 cm3 of CO2
ii) From the data read in 3e(i), moles of CO2 is:
n=
64
1000 d m3
24 d m3 mol−1 =0.0026667 mol
The molar volume of the gas can be determined by gas law given by:
= 0.340 g
100.0869 g mo l−1 =0.003397048mol
nC H 3 COOH= 1d m3 × 50.0 c m3 × 0.500 mol d m−3
1000 c m3 =0.025
The mole ratio of CaCO3: CH3COOH is 1:7, which indicates that CH3COOH was in
excess.
d) Plotting the data, blue dots are data points given and the red line is a straight line of best
fit through the data points.
e) From the plotted graph:
i) O.3 g of CaCO3 will produce 64 cm3 of CO2
ii) From the data read in 3e(i), moles of CO2 is:
n=
64
1000 d m3
24 d m3 mol−1 =0.0026667 mol
The molar volume of the gas can be determined by gas law given by:

PV =nRT V m =nRT
P =0.0026667 mol × 0.08206 L atm mol−1 K−1 × 273 K
1atm
¿ 0.05973968 L=59.73968 c m3
f) A small amount of CaCO3 is first added in acid solution to salute with carbon (IV) oxide.
The graph in figure above, the line of best fit does not pass through the origin since some
CO2is absorbed by water in solution of acid. CO2 is soluble because water molecules are
attracted to these polar areas.
g) Combined gas law is given by:
P1 V 1
T 1
= P2 V 2
T 2
i. Given V 1=24.2 d m3 , T 1=24.0 ° C=297.15 K , P1 =P2 , T 2=18.5° C=291.65
∴ V 2= V 1 T 2
T 1
=24.2 ×291.65
297.15 ¿ 23.75208 d m3
ii. Given
V 1=82.0 cm3 , T1=24.0° C=297.15 K , P1=P2 , T2 =18.5° C=291.65
∴ V 2= V 1 T 2
T 1
=82.0 × 291.65
297.15 ¿ 80.48225 cm3
h) Molar mass calculation
i. Molar volume=24.2 d m3 mol−1 density =1.40 g dm−3
molar mass=1.40 g dm−3 ×24.2 d m3 mo l−1=33.88 g mol−1
ii. If the molecular formula of the gas is XH3
Then X has atomic mass of 33.880−3 ( 1.008 ) =30.856
From periodic table an element with atomic mass of 30.856 is Phosphorus, thus
element x is Phosphorus, P
P =0.0026667 mol × 0.08206 L atm mol−1 K−1 × 273 K
1atm
¿ 0.05973968 L=59.73968 c m3
f) A small amount of CaCO3 is first added in acid solution to salute with carbon (IV) oxide.
The graph in figure above, the line of best fit does not pass through the origin since some
CO2is absorbed by water in solution of acid. CO2 is soluble because water molecules are
attracted to these polar areas.
g) Combined gas law is given by:
P1 V 1
T 1
= P2 V 2
T 2
i. Given V 1=24.2 d m3 , T 1=24.0 ° C=297.15 K , P1 =P2 , T 2=18.5° C=291.65
∴ V 2= V 1 T 2
T 1
=24.2 ×291.65
297.15 ¿ 23.75208 d m3
ii. Given
V 1=82.0 cm3 , T1=24.0° C=297.15 K , P1=P2 , T2 =18.5° C=291.65
∴ V 2= V 1 T 2
T 1
=82.0 × 291.65
297.15 ¿ 80.48225 cm3
h) Molar mass calculation
i. Molar volume=24.2 d m3 mol−1 density =1.40 g dm−3
molar mass=1.40 g dm−3 ×24.2 d m3 mo l−1=33.88 g mol−1
ii. If the molecular formula of the gas is XH3
Then X has atomic mass of 33.880−3 ( 1.008 ) =30.856
From periodic table an element with atomic mass of 30.856 is Phosphorus, thus
element x is Phosphorus, P
1 out of 6
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.


