Specific Heat Capacity of a Solid - Experiment and Theory
VerifiedAdded on 2022/11/11
|11
|1615
|317
AI Summary
This experiment observes the transfer of energy when there is an imbalance of temperature between two substances. The experiment is an investigation of the relationship between the energy needed to changes the temperature of materials and the mass.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

THERMODYNAMIC & FLUID MECHANICS
By Name
Course
Instructor
Institution
Location
Date
By Name
Course
Instructor
Institution
Location
Date
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Introduction
This experiment observes the transfer of energy when there is an imbalance of temperature
between two substances. The experiment is an investigation of the relationship between the
energy needed to changes the temperature of materials and the mass.
Theory
The specific heat capacity of a solid or a material defines the amount of heat energy for every
unit mass that is needed to increase the temperature of 1kg of the material or substance by 1
Kelvin. Transfer of heat occurs where there is an imbalance in the temperature. The specific heat
capacity of any substance is the heat for every unit mass hence heart capacity=mass*specific
heat. The specific heat is basically a measure of thermal insensitivity of substances to the
addition of more energy to it. The water equivalent of a substance is the mass of water that
would need the same amount of heat as the substance to increase the temperature through a
single Celsius (Ferrer, Barreneche, Solé, Martorell & Cabeza, 2017).
The method of mixtures has for some time been used in the determination of the specific heat
capacity of bodies. The method utilizes the principles that if two bodies at varied temperatures
engage in heat exchange, the amount of heat that is lost by the body having a higher temperature
is the same as the one gained by the body that has lower temperature and certain intermediate
equilibrium is finally attained. This statement holds as long as there is loss or gain of heat from
or to the surrounding. The role of the calorimeter in such a set-up is to ensure there is no loss of
heat to the environment. There are mainly three methods of transfer of heat including
conduction, radiation as well as convention.
This experiment observes the transfer of energy when there is an imbalance of temperature
between two substances. The experiment is an investigation of the relationship between the
energy needed to changes the temperature of materials and the mass.
Theory
The specific heat capacity of a solid or a material defines the amount of heat energy for every
unit mass that is needed to increase the temperature of 1kg of the material or substance by 1
Kelvin. Transfer of heat occurs where there is an imbalance in the temperature. The specific heat
capacity of any substance is the heat for every unit mass hence heart capacity=mass*specific
heat. The specific heat is basically a measure of thermal insensitivity of substances to the
addition of more energy to it. The water equivalent of a substance is the mass of water that
would need the same amount of heat as the substance to increase the temperature through a
single Celsius (Ferrer, Barreneche, Solé, Martorell & Cabeza, 2017).
The method of mixtures has for some time been used in the determination of the specific heat
capacity of bodies. The method utilizes the principles that if two bodies at varied temperatures
engage in heat exchange, the amount of heat that is lost by the body having a higher temperature
is the same as the one gained by the body that has lower temperature and certain intermediate
equilibrium is finally attained. This statement holds as long as there is loss or gain of heat from
or to the surrounding. The role of the calorimeter in such a set-up is to ensure there is no loss of
heat to the environment. There are mainly three methods of transfer of heat including
conduction, radiation as well as convention.

When a solid gains heat energy, the total internal energy is raised where the total internal energy
defines the sum of the potential as well as kinetic energies of all molecules that are found in the
substance. The measure of the average kinetic energy defines the temperature of the molecules
found in substances of which the two are directly proportional. The temperature of a substance is
greater when the average kinetic energy is as well greater (Ferrer et al., 2017). When two various
substances are supplied with the same quantity of heat, there may be a significant change in their
temperatures meaning the flow of heat as well as a change in temperature in an object has some
proportionality constant relationship that is unique to the specific substance. The proportionality
constant is defined as the specific heat, c, of the substance which is often measured in J/kg⁰C
Q=mc∆T
The second law of thermodynamics provides that when two objects are at varying temperatures
and are brought to touch each other, there is a flow of heat from warmer to cooler object, In case
the objects are kept in contact for adequate time, an equilibrium will be attained at the same final
temperatures, Tf. The first law of thermodynamics, on the other hand, states that thermal energy
is conserved meaning the same amount of heat gets out of the warmer object as flows into the
colder object (Sekhar & Sharma, 2015)
Heat lost=heat gained
In the experiment, a heated solid having a known mass as well as temperature is dropped inside a
calorimeter that contains a known mass of cold water. Measurement of the equilibrium
temperature is taken. The extent of the heat lost by solid has to be equivalent to the magnitude of
the heat that is gained by the water as well as the calorimeter with a stirrer.
Aim: To determine the specific heat capacity of a solid
defines the sum of the potential as well as kinetic energies of all molecules that are found in the
substance. The measure of the average kinetic energy defines the temperature of the molecules
found in substances of which the two are directly proportional. The temperature of a substance is
greater when the average kinetic energy is as well greater (Ferrer et al., 2017). When two various
substances are supplied with the same quantity of heat, there may be a significant change in their
temperatures meaning the flow of heat as well as a change in temperature in an object has some
proportionality constant relationship that is unique to the specific substance. The proportionality
constant is defined as the specific heat, c, of the substance which is often measured in J/kg⁰C
Q=mc∆T
The second law of thermodynamics provides that when two objects are at varying temperatures
and are brought to touch each other, there is a flow of heat from warmer to cooler object, In case
the objects are kept in contact for adequate time, an equilibrium will be attained at the same final
temperatures, Tf. The first law of thermodynamics, on the other hand, states that thermal energy
is conserved meaning the same amount of heat gets out of the warmer object as flows into the
colder object (Sekhar & Sharma, 2015)
Heat lost=heat gained
In the experiment, a heated solid having a known mass as well as temperature is dropped inside a
calorimeter that contains a known mass of cold water. Measurement of the equilibrium
temperature is taken. The extent of the heat lost by solid has to be equivalent to the magnitude of
the heat that is gained by the water as well as the calorimeter with a stirrer.
Aim: To determine the specific heat capacity of a solid

Materials & Methods
Equipment
Calorimeter
Thermometer
Stirrer
Lid
Funnel
Metal
Procedure
1. A beaker is half filled with water and put on an electric stove
2. A piece of aluminium metal mass is weighed and recorded
3. 100 ml of water is measured at room temperature and added into a calorimeter. The mass
of the water is determined.
4. The temperature of the water inside the calorimeter is recorded
5. Aluminium metal and thermometer are suspended in boiling water for approximately 120
seconds and the temperature recorded. This is treated as an initial temperature of
aluminium metal
6. The metal is quickly but carefully transferred into the calorimeter and then stirred. The
temperature of the calorimeter content is recorded at intervals of 10 seconds until no
further change in the temperature
7. The specific heat capacity of aluminium metal is calculated
8. The above procedure is repeated with a piece of copper metal
Equipment
Calorimeter
Thermometer
Stirrer
Lid
Funnel
Metal
Procedure
1. A beaker is half filled with water and put on an electric stove
2. A piece of aluminium metal mass is weighed and recorded
3. 100 ml of water is measured at room temperature and added into a calorimeter. The mass
of the water is determined.
4. The temperature of the water inside the calorimeter is recorded
5. Aluminium metal and thermometer are suspended in boiling water for approximately 120
seconds and the temperature recorded. This is treated as an initial temperature of
aluminium metal
6. The metal is quickly but carefully transferred into the calorimeter and then stirred. The
temperature of the calorimeter content is recorded at intervals of 10 seconds until no
further change in the temperature
7. The specific heat capacity of aluminium metal is calculated
8. The above procedure is repeated with a piece of copper metal
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

ρwater =997 kg
m3 , cwater= 4190J / kg° K
Results
Temperature of water=23.8⁰C
The temperature of boiling water=101.1⁰C
Mass of aluminium=58.03g
Temperature of water=23.8⁰C
The temperature of boiling water/mass of copper=101.1⁰C/200.31g
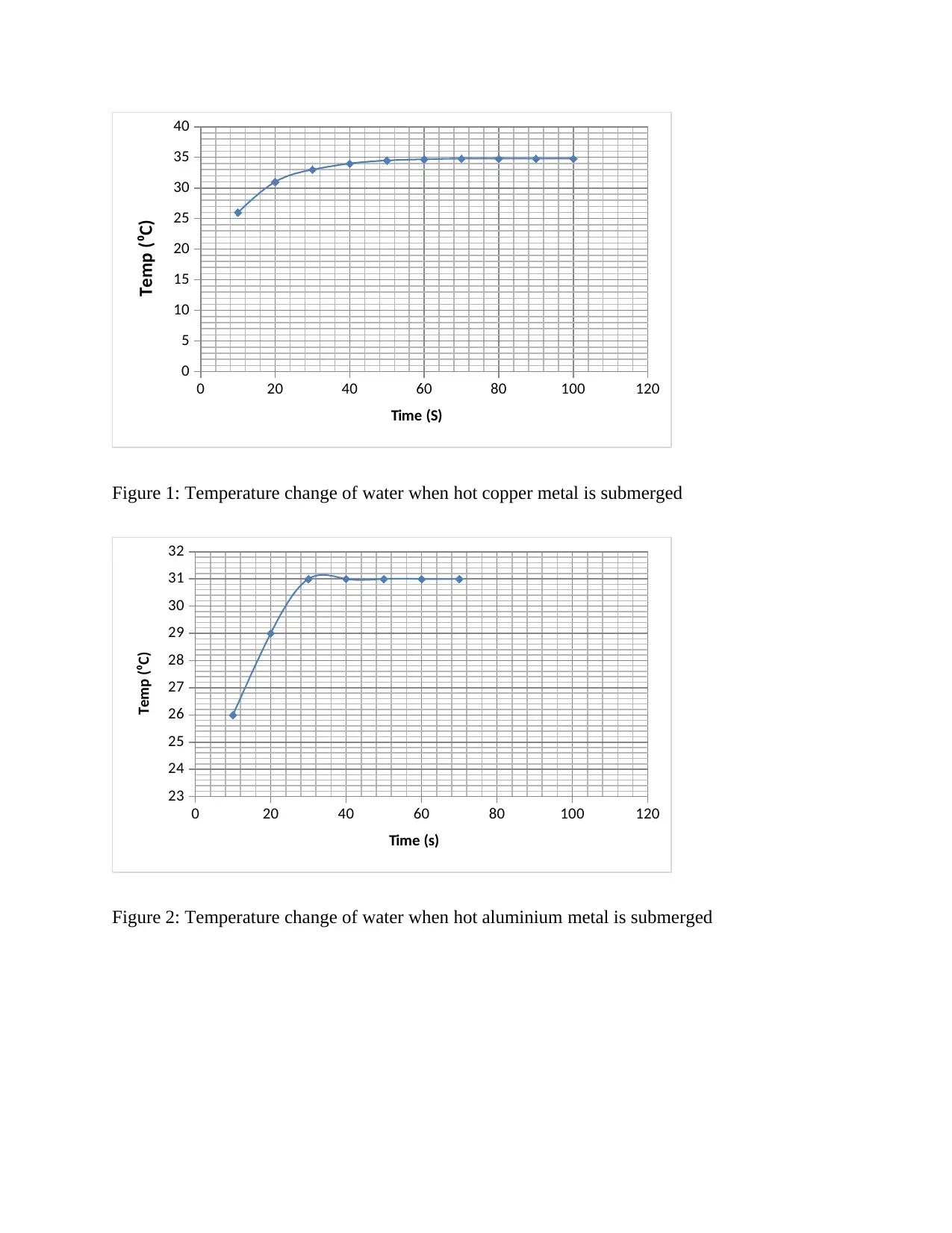
Time (s) Copper Aluminium
10 26 26
20 31 29
30 33 31
40 34 31
50 34.5 31
60 34.7 31
70 34.8 31
80 34.8
90 34.8
100 34.8
Table 1: Temperature change of water when hot aluminium & copper metals are submerged
m3 , cwater= 4190J / kg° K
Results
Temperature of water=23.8⁰C
The temperature of boiling water=101.1⁰C
Mass of aluminium=58.03g
Temperature of water=23.8⁰C
The temperature of boiling water/mass of copper=101.1⁰C/200.31g
Time (s) Copper Aluminium
10 26 26
20 31 29
30 33 31
40 34 31
50 34.5 31
60 34.7 31
70 34.8 31
80 34.8
90 34.8
100 34.8
Table 1: Temperature change of water when hot aluminium & copper metals are submerged
0 20 40 60 80 100 120
0
5
10
15
20
25
30
35
40
Time (S)
Temp (⁰C)
Figure 1: Temperature change of water when hot copper metal is submerged
0 20 40 60 80 100 120
23
24
25
26
27
28
29
30
31
32
Time (s)
Temp (⁰C)
Figure 2: Temperature change of water when hot aluminium metal is submerged
0
5
10
15
20
25
30
35
40
Time (S)
Temp (⁰C)
Figure 1: Temperature change of water when hot copper metal is submerged
0 20 40 60 80 100 120
23
24
25
26
27
28
29
30
31
32
Time (s)
Temp (⁰C)
Figure 2: Temperature change of water when hot aluminium metal is submerged

Calculations
Specific heat capacity of aluminium is calculated as follows
Q=mc∆T
Where
Q is the heat change
m=mass
c=specific hear capacity
∆T=change in temperature
Heat lost by metal=Heat gained by the water
Heat lost by metal=mAlcAl∆TAl=0.05803*C*(101.1-34.8) =3.847CAl
Heat gained by water=mwCw∆Tw=0.1*4190*(34.8-23.8) =4609
CAl=4609/3.847=1198.1 Jkg-1K-1
C=1198.1 Jkg-1K-1
Specific heat capacity of copper is calculated as follows
Heat lost by metal=Heat gained by the water
Heat lost by metal=mCucCu∆TCu=0.20031*C*(101.1-31) =14.04CAl
Heat gained by water=mwCw∆Tw=0.1*4190*(31-23.8) =3016.8
CCu=3016.8/14.04=214.87 Jkg-1K-1
Specific heat capacity of aluminium is calculated as follows
Q=mc∆T
Where
Q is the heat change
m=mass
c=specific hear capacity
∆T=change in temperature
Heat lost by metal=Heat gained by the water
Heat lost by metal=mAlcAl∆TAl=0.05803*C*(101.1-34.8) =3.847CAl
Heat gained by water=mwCw∆Tw=0.1*4190*(34.8-23.8) =4609
CAl=4609/3.847=1198.1 Jkg-1K-1
C=1198.1 Jkg-1K-1
Specific heat capacity of copper is calculated as follows
Heat lost by metal=Heat gained by the water
Heat lost by metal=mCucCu∆TCu=0.20031*C*(101.1-31) =14.04CAl
Heat gained by water=mwCw∆Tw=0.1*4190*(31-23.8) =3016.8
CCu=3016.8/14.04=214.87 Jkg-1K-1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

C=214.87 Jkg-1K-1
Discussion and Analysis
Q1. Instruments that can be used for accurate measurement of temperature and their advantages
A liquid-in-glass thermometer is composed of a glass bulb that contains a specific liquid
commonly mercury which expands and contracts in relation to response in changes in the
temperature thus showing the temperature as position on the scale of the stem. An advantage of
this instrument is that it does not require a power source, easy to use as well as repeatable
(Sekhar & Sharma, 2015).
Radiation Thermometry is composed of a series of optics that concentrate infrared light onto a
special electronic detector that is often a semiconductor that in turn generates an electric current
that is proportional to the intensity of infrared radiation intensity. Radiation thermometers can
measure the temperature of objects at a distance and this is a key advantage.
Constant-Volume Gas thermometer is composed of a container having a fixed gas amount within
and works on the principle of variations in gas pressure are proportional to gas temperature
changes. The pressure is detected by a pressure sensor inside and the value is converted to a
measurement of temperature by calibration electronics. The instrument is linear and accurate as
of the major advantages (Sekhar & Sharma, 2015).
Q2. The method may be refined by doing the experiment using an insulated beaker that would
prevent losing heat as a result of heating the beaker and through evaporation.
Q3. Not all the energy that is lost by the metal is gained by the heat as some is lost through
evaporation and in heating the beaker. The metal should not touch the beaker as there would
Discussion and Analysis
Q1. Instruments that can be used for accurate measurement of temperature and their advantages
A liquid-in-glass thermometer is composed of a glass bulb that contains a specific liquid
commonly mercury which expands and contracts in relation to response in changes in the
temperature thus showing the temperature as position on the scale of the stem. An advantage of
this instrument is that it does not require a power source, easy to use as well as repeatable
(Sekhar & Sharma, 2015).
Radiation Thermometry is composed of a series of optics that concentrate infrared light onto a
special electronic detector that is often a semiconductor that in turn generates an electric current
that is proportional to the intensity of infrared radiation intensity. Radiation thermometers can
measure the temperature of objects at a distance and this is a key advantage.
Constant-Volume Gas thermometer is composed of a container having a fixed gas amount within
and works on the principle of variations in gas pressure are proportional to gas temperature
changes. The pressure is detected by a pressure sensor inside and the value is converted to a
measurement of temperature by calibration electronics. The instrument is linear and accurate as
of the major advantages (Sekhar & Sharma, 2015).
Q2. The method may be refined by doing the experiment using an insulated beaker that would
prevent losing heat as a result of heating the beaker and through evaporation.
Q3. Not all the energy that is lost by the metal is gained by the heat as some is lost through
evaporation and in heating the beaker. The metal should not touch the beaker as there would

likely be chances of heat transfer that would, in turn, interfere with the accuracy of the
experiment. During the calculation, it is assumed there are not heat losses or gains to and from
the environment.
Q4. It was assumed that the water used in the experiment was not pure and hence could not have
a standard boiling point. The measurements of the true temperature of the water were taken and
noted to be 101.1⁰C. The metal cylinder as well lost heat to the environment.
Q5. Properties of materials that make them suitable for solar heater applications
High conductivity of heat
Resistance to water corrosion
Atmospheric corrosion resistance
Q6. Cv= ( ∂U
∂ V )T
. Differentiating the equation with respect to volume:
( ∂ Cv
∂ V )T
= ( ∂
∂ V ( ∂ U
∂T )V )T
=
( ∂
∂ T ( ∂ U
∂T ) T )V
Using the relation provided in the question, this can be written as:
( ∂ Cv
∂ V )T
= ( ∂
∂ V ( ∂ U
∂T )V )T
=( ∂ (−P+T (∂ P /∂T ))
∂T )V
=T ( ∂2 P
∂ T2 )V
The various equations of state are then considered:
For an ideal gas. P=nRT/V where the second derivative of pressure, as shown above, is zero
hence ( ∂ Cv
∂ V )T
=0
experiment. During the calculation, it is assumed there are not heat losses or gains to and from
the environment.
Q4. It was assumed that the water used in the experiment was not pure and hence could not have
a standard boiling point. The measurements of the true temperature of the water were taken and
noted to be 101.1⁰C. The metal cylinder as well lost heat to the environment.
Q5. Properties of materials that make them suitable for solar heater applications
High conductivity of heat
Resistance to water corrosion
Atmospheric corrosion resistance
Q6. Cv= ( ∂U
∂ V )T
. Differentiating the equation with respect to volume:
( ∂ Cv
∂ V )T
= ( ∂
∂ V ( ∂ U
∂T )V )T
=
( ∂
∂ T ( ∂ U
∂T ) T )V
Using the relation provided in the question, this can be written as:
( ∂ Cv
∂ V )T
= ( ∂
∂ V ( ∂ U
∂T )V )T
=( ∂ (−P+T (∂ P /∂T ))
∂T )V
=T ( ∂2 P
∂ T2 )V
The various equations of state are then considered:
For an ideal gas. P=nRT/V where the second derivative of pressure, as shown above, is zero
hence ( ∂ Cv
∂ V )T
=0

For a van der Waals gas,P= nRT
V −nb − n2 a
V 2 , differentiating P with respect to T once more gives
nR/ (V −nb) which is not dependent on T and thus ( ∂ Cv
∂ V )T
=0
Differentiating P for the second time with respect to T give zero and thus ( ∂ Cv
∂ V )T
=0
V −nb − n2 a
V 2 , differentiating P with respect to T once more gives
nR/ (V −nb) which is not dependent on T and thus ( ∂ Cv
∂ V )T
=0
Differentiating P for the second time with respect to T give zero and thus ( ∂ Cv
∂ V )T
=0
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

References
Ferrer, G., Barreneche, C., Solé, A., Martorell, I., & Cabeza, L. F. (2017). New proposed
methodology for specific heat capacity determination of materials for thermal energy
storage (TES) by DSC. Journal of Energy Storage, 11, 1-6.
Sekhar, Y. R., & Sharma, K. V. (2015). Study of viscosity and specific heat capacity
characteristics of water-based Al2O3 nanofluids at low particle concentrations. Journal
of Experimental Nanoscience, 10(2), 86-102
Ferrer, G., Barreneche, C., Solé, A., Martorell, I., & Cabeza, L. F. (2017). New proposed
methodology for specific heat capacity determination of materials for thermal energy
storage (TES) by DSC. Journal of Energy Storage, 11, 1-6.
Sekhar, Y. R., & Sharma, K. V. (2015). Study of viscosity and specific heat capacity
characteristics of water-based Al2O3 nanofluids at low particle concentrations. Journal
of Experimental Nanoscience, 10(2), 86-102
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.





