Biology: Investigating Tissue Staining Techniques & Disease Diagnosis
VerifiedAdded on 2023/05/30
|9
|1997
|368
Report
AI Summary
This report provides an overview of tissue staining techniques used in biology and medicine for enhancing contrast in microscopic images. It discusses simple, differential, and special staining methods, highlighting their applications in visualizing cellular structures and diagnosing diseases such as Alzheimer's and Parkinson's. Specific stains like Congo red for detecting amyloid plaques in Alzheimer's disease and silver staining for identifying Lewy bodies in Parkinson's disease are examined. The report includes observations from stained tissue samples, showcasing the importance of histology in understanding cell and organ functions, and emphasizes the role of tissue staining in disease diagnosis. Desklib is a platform where students can access this and other solved assignments for study purposes.

Running head: BIOLOGY 1
Biology
Student’s Name
Institutional Affiliation
Biology
Student’s Name
Institutional Affiliation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

BIOLOGY 2
Purpose
Tissue staining is a supplemental method utilized in microscopy for facilitating contrast
in the microscopic picture. Stains along with dyes are utilized regularly in biology along with
medicine for underlining structures in biological tissues for visualization (Alturkistani,
Tashkandi & Mohammedsaleh, 2016). The purpose of tissue staining is to highlight outlines of
cells since some stains can penetrate cell walls to highlight cell elements which assist scientists
in visualizing metabolic processes. Also, it helps to differentiate between dead cells and live cells
together with enabling scientists to count the number of cells of a specific type in particular
biomass.
Introduction
Types of staining are simple staining, differential staining, and special or structural
straining. Simple staining entails immersing the sample in dye solution proceeded by rinsing and
observations. This is a one-step approach of using only one dye, and it is utilized in studying
morphology better and demonstrating the nature of the cellular contents of the exudates along
with exploring the intracellular location of the bacteria. Basic stains like Gram safranin, Gram
crystal violet or methylene blue are useful for staining most bacteria (Beech, Noimark, Page,
Noor, Allan & Parkin, 2015).
In differential staining, two or more stains are used and let the cells be classified into
different types or groups. Other than letting the observation of cell morphology or the shape, this
type of staining frequently give more details about the characteristics of the cell wall such as its
thickness. Its examples are Gram stain used for differentiating bacteria into two groups and the
acid-fast stain which distinguishes species of Mycobacterium from other bacteria. Special
Purpose
Tissue staining is a supplemental method utilized in microscopy for facilitating contrast
in the microscopic picture. Stains along with dyes are utilized regularly in biology along with
medicine for underlining structures in biological tissues for visualization (Alturkistani,
Tashkandi & Mohammedsaleh, 2016). The purpose of tissue staining is to highlight outlines of
cells since some stains can penetrate cell walls to highlight cell elements which assist scientists
in visualizing metabolic processes. Also, it helps to differentiate between dead cells and live cells
together with enabling scientists to count the number of cells of a specific type in particular
biomass.
Introduction
Types of staining are simple staining, differential staining, and special or structural
straining. Simple staining entails immersing the sample in dye solution proceeded by rinsing and
observations. This is a one-step approach of using only one dye, and it is utilized in studying
morphology better and demonstrating the nature of the cellular contents of the exudates along
with exploring the intracellular location of the bacteria. Basic stains like Gram safranin, Gram
crystal violet or methylene blue are useful for staining most bacteria (Beech, Noimark, Page,
Noor, Allan & Parkin, 2015).
In differential staining, two or more stains are used and let the cells be classified into
different types or groups. Other than letting the observation of cell morphology or the shape, this
type of staining frequently give more details about the characteristics of the cell wall such as its
thickness. Its examples are Gram stain used for differentiating bacteria into two groups and the
acid-fast stain which distinguishes species of Mycobacterium from other bacteria. Special

BIOLOGY 3
staining isolates the particular part of microbes. Malachite green is utilized with heat to force the
dye into the cells and provide the color. Safranin, a counterstain is therefore utilized in providing
color to the non-spore forming microbes, and at the end of the process spores stain green, and
other cells stain red (Ashikuzzaman, Shahriyar, Lijon, Rahman, Hassan & Asif, 2015).
The Nissl-staining technique is based on the interactions of critical dyes like cresyl violet,
thionine, toluidine blue, methylene blue or any with the nucleic acid content of cells. This
technique detects the nissl body in the cytoplasm of neurons on paraformaldehyde fixed frozen
or vibratome tissue parts which are stained purple-blue. The organelles stained by Nissl are
neurons and glial cells (García-Cabezas, John, Barbas & Zikopoulos, 2016).
Congo red is a different stain related to Alzheimer's disease. Beta-amyloid plaques can be
detected using Congo red staining, and their presence along with neurofibrillary tangles is vital
for the diagnosis of Alzheimer disease (Ho, Troncoso, Knox, Stark & Eberhart, 2014).
Symptoms in the early stages of Alzheimer are impairment of disorientation, declarative memory
and loss of context. The primary brain structures which support these functions include the
medial temporal lobe more so the hippocampal formation and adjacent cortex. Specifically, the
CA1 hippocampal region is critical in spatial orientation, learning and distinct memory functions
like the retrieval of remote episodic memory along with the strength of developed memories
(Danielson, Zaremba, Kaifosh, Bowler, Ladow & Losonczy, 2016).
In the histology world, Congo red stain is used to stain amyloid which is an exceptionally
folded fibrillar protein which deposits in extracellular spaces in organs under particular
pathological states (Ho, Troncoso, Knox, Stark & Eberhart, 2014). As this protein multiplies it
increasingly restores the standard tissue components and finally leads to loss of function of
staining isolates the particular part of microbes. Malachite green is utilized with heat to force the
dye into the cells and provide the color. Safranin, a counterstain is therefore utilized in providing
color to the non-spore forming microbes, and at the end of the process spores stain green, and
other cells stain red (Ashikuzzaman, Shahriyar, Lijon, Rahman, Hassan & Asif, 2015).
The Nissl-staining technique is based on the interactions of critical dyes like cresyl violet,
thionine, toluidine blue, methylene blue or any with the nucleic acid content of cells. This
technique detects the nissl body in the cytoplasm of neurons on paraformaldehyde fixed frozen
or vibratome tissue parts which are stained purple-blue. The organelles stained by Nissl are
neurons and glial cells (García-Cabezas, John, Barbas & Zikopoulos, 2016).
Congo red is a different stain related to Alzheimer's disease. Beta-amyloid plaques can be
detected using Congo red staining, and their presence along with neurofibrillary tangles is vital
for the diagnosis of Alzheimer disease (Ho, Troncoso, Knox, Stark & Eberhart, 2014).
Symptoms in the early stages of Alzheimer are impairment of disorientation, declarative memory
and loss of context. The primary brain structures which support these functions include the
medial temporal lobe more so the hippocampal formation and adjacent cortex. Specifically, the
CA1 hippocampal region is critical in spatial orientation, learning and distinct memory functions
like the retrieval of remote episodic memory along with the strength of developed memories
(Danielson, Zaremba, Kaifosh, Bowler, Ladow & Losonczy, 2016).
In the histology world, Congo red stain is used to stain amyloid which is an exceptionally
folded fibrillar protein which deposits in extracellular spaces in organs under particular
pathological states (Ho, Troncoso, Knox, Stark & Eberhart, 2014). As this protein multiplies it
increasingly restores the standard tissue components and finally leads to loss of function of
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

BIOLOGY 4
essential organs and eventually death. Amyloid is same in structure to cellulose hence it behaves
the same way in its chemical reactions. This is a linear molecule that permits amine and azo
groups of the dye to generate hydrogen bonds with same hydroxyl radicals of the amyloid (Ho,
Troncoso, Knox, Stark & Eberhart, 2014).
When it is explored in hematoxylin and eosin stained parts of tissue, amyloid emerges as
an amorphous, glassy, eosinophilic substance. Because this can be confused with some other
elements Congo red is required to recognize it. Moreover, when studied using frequent bright
field microscopy, Congo red-stained amyloid emerges pale orange-red (Ho, Troncoso, Knox,
Stark & Eberhart, 2014). Consequently, the bright field aspect alone is not diagnostic for
amyloid since small deposits may be challenging to view. Therefore, Congo red-stained tissue
sections should be surveyed under polarized light permitting the characteristic apple-green
birefringence to be observed which is diagnostic for the amyloid presence. Congo red stain
remains the standard gold test utilized by pathologists to discover amyloid in tissues of
individuals with these states possibly the Alzheimer’s disease. Congo red stains amyloidosis,
plant and fungi cell walls along with Gram-negative bacterial outer membrane (Costa et al.,
2015).
Silver staining is related to Parkinson’s disease whereby the silver stained sections
demonstrate all Lewy bodies along with Lewy neuritis (Saito et al., 2016). Large Lewy bodies
can be detected with acidic dyes, but H&E routine stains are not adequately sensitive to discover
all the Parkinson's disease associated alterations. Presently, the presynaptic protein alpha-
synucleic has been reported to be present in all kinds of Lewy bodies and Lewy neuritis, and
immunocytochemical demonstration of alpha-synucleic is regarded as the gold standard, reliable
essential organs and eventually death. Amyloid is same in structure to cellulose hence it behaves
the same way in its chemical reactions. This is a linear molecule that permits amine and azo
groups of the dye to generate hydrogen bonds with same hydroxyl radicals of the amyloid (Ho,
Troncoso, Knox, Stark & Eberhart, 2014).
When it is explored in hematoxylin and eosin stained parts of tissue, amyloid emerges as
an amorphous, glassy, eosinophilic substance. Because this can be confused with some other
elements Congo red is required to recognize it. Moreover, when studied using frequent bright
field microscopy, Congo red-stained amyloid emerges pale orange-red (Ho, Troncoso, Knox,
Stark & Eberhart, 2014). Consequently, the bright field aspect alone is not diagnostic for
amyloid since small deposits may be challenging to view. Therefore, Congo red-stained tissue
sections should be surveyed under polarized light permitting the characteristic apple-green
birefringence to be observed which is diagnostic for the amyloid presence. Congo red stain
remains the standard gold test utilized by pathologists to discover amyloid in tissues of
individuals with these states possibly the Alzheimer’s disease. Congo red stains amyloidosis,
plant and fungi cell walls along with Gram-negative bacterial outer membrane (Costa et al.,
2015).
Silver staining is related to Parkinson’s disease whereby the silver stained sections
demonstrate all Lewy bodies along with Lewy neuritis (Saito et al., 2016). Large Lewy bodies
can be detected with acidic dyes, but H&E routine stains are not adequately sensitive to discover
all the Parkinson's disease associated alterations. Presently, the presynaptic protein alpha-
synucleic has been reported to be present in all kinds of Lewy bodies and Lewy neuritis, and
immunocytochemical demonstration of alpha-synucleic is regarded as the gold standard, reliable
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

BIOLOGY 5
recognition of the whole spectrum of the Parkinson's disease-related cytoskeletal alterations.
Silver stains mitochondria organelles.
Results/ Observations
Figure 1 Spherulites observed in Alzheimer's hippocampal tissue stained with Congo red and
hematoxylin
a) Congo-red-stained spherulite giving apple-green birefringence under progressively
crossed polarizers.
(a-c) Typical of the senile plaques observed in Alzheimer's disease.
d) Spherulite structures (solid arrow-heads) in the same tissue section without either the
strong affinity for Congo red.
e) apple-green birefringence under crossed polarizers. In all parts, the unstained
spherulites were distributed in the granule cell layer (vertical arrow) of the dentate gyrus (DG)
and a surrounding band in Ammon's horn (CA) within the pyramidal cell layer.
f) The hippocampal section with a lighter Congo red stain, hematoxylin-positive cells
(vertical arrow) and the spherulites which also notably lack affinity for hematoxylin (solid arrow-
heads) shown under partially crossed polarizers.
g) The same region under fully crossed polarizers.
recognition of the whole spectrum of the Parkinson's disease-related cytoskeletal alterations.
Silver stains mitochondria organelles.
Results/ Observations
Figure 1 Spherulites observed in Alzheimer's hippocampal tissue stained with Congo red and
hematoxylin
a) Congo-red-stained spherulite giving apple-green birefringence under progressively
crossed polarizers.
(a-c) Typical of the senile plaques observed in Alzheimer's disease.
d) Spherulite structures (solid arrow-heads) in the same tissue section without either the
strong affinity for Congo red.
e) apple-green birefringence under crossed polarizers. In all parts, the unstained
spherulites were distributed in the granule cell layer (vertical arrow) of the dentate gyrus (DG)
and a surrounding band in Ammon's horn (CA) within the pyramidal cell layer.
f) The hippocampal section with a lighter Congo red stain, hematoxylin-positive cells
(vertical arrow) and the spherulites which also notably lack affinity for hematoxylin (solid arrow-
heads) shown under partially crossed polarizers.
g) The same region under fully crossed polarizers.
BIOLOGY 6
Levels of beta-amyloid are generally low in the cerebrospinal fluid of Alzheimer's
patients and cerebral amyloid angiopathic patients (Merlini, Wanner & Nitsch, 2016).
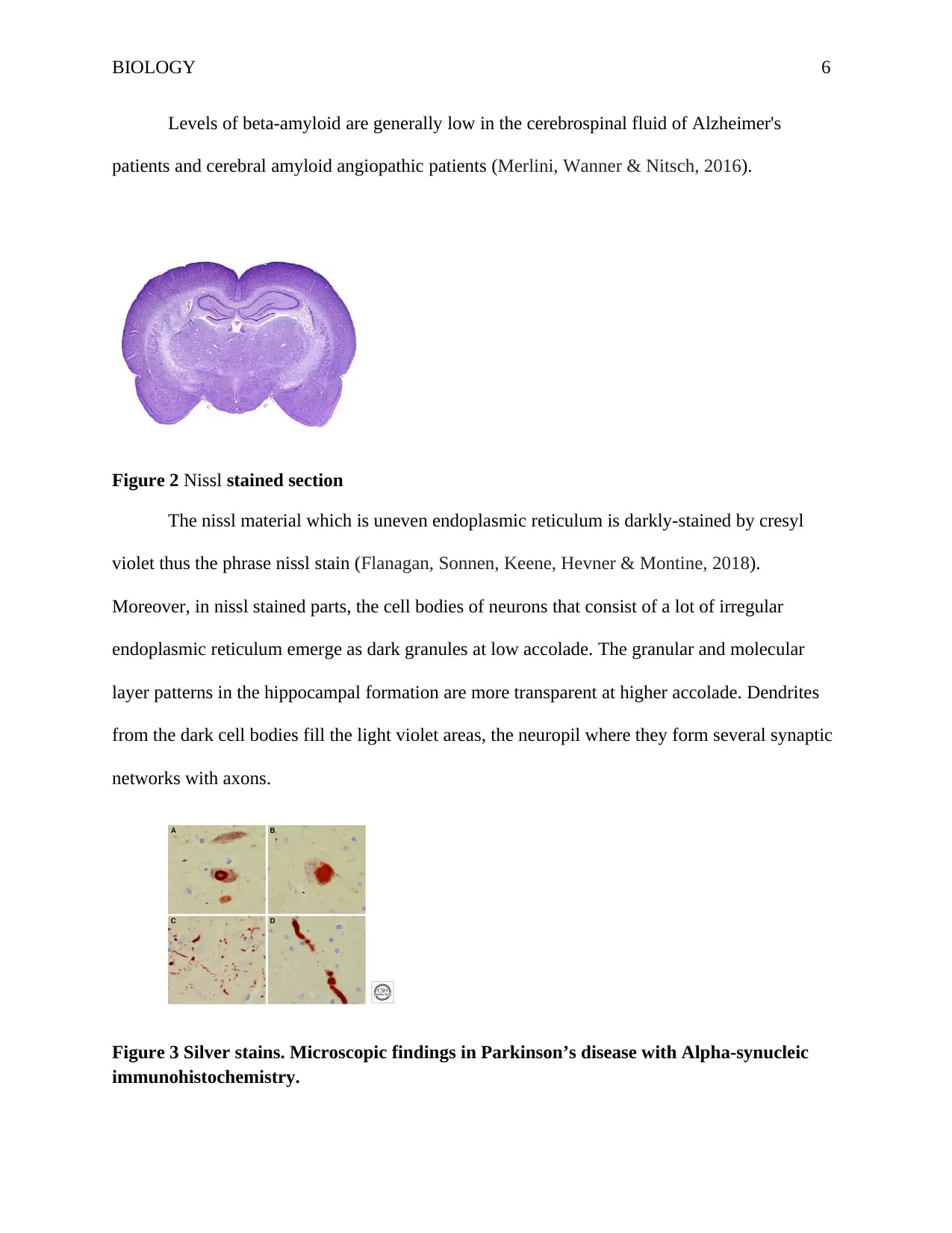
Figure 2 Nissl stained section
The nissl material which is uneven endoplasmic reticulum is darkly-stained by cresyl
violet thus the phrase nissl stain (Flanagan, Sonnen, Keene, Hevner & Montine, 2018).
Moreover, in nissl stained parts, the cell bodies of neurons that consist of a lot of irregular
endoplasmic reticulum emerge as dark granules at low accolade. The granular and molecular
layer patterns in the hippocampal formation are more transparent at higher accolade. Dendrites
from the dark cell bodies fill the light violet areas, the neuropil where they form several synaptic
networks with axons.
Figure 3 Silver stains. Microscopic findings in Parkinson’s disease with Alpha-synucleic
immunohistochemistry.
Levels of beta-amyloid are generally low in the cerebrospinal fluid of Alzheimer's
patients and cerebral amyloid angiopathic patients (Merlini, Wanner & Nitsch, 2016).
Figure 2 Nissl stained section
The nissl material which is uneven endoplasmic reticulum is darkly-stained by cresyl
violet thus the phrase nissl stain (Flanagan, Sonnen, Keene, Hevner & Montine, 2018).
Moreover, in nissl stained parts, the cell bodies of neurons that consist of a lot of irregular
endoplasmic reticulum emerge as dark granules at low accolade. The granular and molecular
layer patterns in the hippocampal formation are more transparent at higher accolade. Dendrites
from the dark cell bodies fill the light violet areas, the neuropil where they form several synaptic
networks with axons.
Figure 3 Silver stains. Microscopic findings in Parkinson’s disease with Alpha-synucleic
immunohistochemistry.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

BIOLOGY 7
A) A normal brainstem type Lewy body
B) a pale staining cortical type Lewy body.
C) Lewy neuritis in CA2 sector of hippocampus
D) Intraneuritic Lewy bodies in medulla.
Conclusion
Tissue staining helps to highlight structures in biological tissues to observe. There are
different staining types of which depend on the region being seen and the kind of disease
associated with it. The association between the cell and organ functioning is reflected in tissue
organization observed under the microscopy thus histology endorses the study of cell biology at
every level. Also, histology is very essential in diagnosing a disease.
A) A normal brainstem type Lewy body
B) a pale staining cortical type Lewy body.
C) Lewy neuritis in CA2 sector of hippocampus
D) Intraneuritic Lewy bodies in medulla.
Conclusion
Tissue staining helps to highlight structures in biological tissues to observe. There are
different staining types of which depend on the region being seen and the kind of disease
associated with it. The association between the cell and organ functioning is reflected in tissue
organization observed under the microscopy thus histology endorses the study of cell biology at
every level. Also, histology is very essential in diagnosing a disease.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

BIOLOGY 8
References
Alturkistani, H. A., Tashkandi, F. M., & Mohammedsaleh, Z. M. (2016). Histological stains: A
literature review and case study. Global journal of health science, 8(3), 72.
Ashikuzzaman, M., Shahriyar, S., Lijon, M. B., Rahman, M. A., Hassan, M. M., & Asif, A. A.
(2015). An investigation on heavy metal tolerance properties of bacteria isolated from
textile effluent. Journal of Biodiversity and Environmental Sciences, 7(6), 62-71.
Beech, S. J., Noimark, S., Page, K., Noor, N., Allan, E., & Parkin, I. P. (2015). Incorporation of
crystal violet, methylene blue, and safranin O into a copolymer emulsion; the
development of a novel antimicrobial paint. RSC Advances, 5(33), 26364-26375.
Costa, T. R., Felisberto-Rodrigues, C., Meir, A., Prevost, M. S., Redzej, A., Trokter, M., &
Waksman, G. (2015). Secretion systems in Gram-negative bacteria: structural and
mechanistic insights. Nature Reviews Microbiology, 13(6), 343.
Danielson, N. B., Zaremba, J. D., Kaifosh, P., Bowler, J., Ladow, M., & Losonczy, A. (2016).
Sublayer-specific coding dynamics during spatial navigation and learning in hippocampal
area CA1. Neuron, 91(3), 652-665.
Flanagan, M., Sonnen, J. A., Keene, C. D., Hevner, R. F., & Montine, T. J. (2018). Molecular
Basis of Diseases of the Nervous System. In Molecular Pathology (Second Edition)(pp.
651-690).
García-Cabezas, M. Á., John, Y. J., Barbas, H., & Zikopoulos, B. (2016). The distinction of
neurons, glia and endothelial cells in the cerebral cortex: an algorithm based on
cytological features. Frontiers in neuroanatomy, 10, 107.
References
Alturkistani, H. A., Tashkandi, F. M., & Mohammedsaleh, Z. M. (2016). Histological stains: A
literature review and case study. Global journal of health science, 8(3), 72.
Ashikuzzaman, M., Shahriyar, S., Lijon, M. B., Rahman, M. A., Hassan, M. M., & Asif, A. A.
(2015). An investigation on heavy metal tolerance properties of bacteria isolated from
textile effluent. Journal of Biodiversity and Environmental Sciences, 7(6), 62-71.
Beech, S. J., Noimark, S., Page, K., Noor, N., Allan, E., & Parkin, I. P. (2015). Incorporation of
crystal violet, methylene blue, and safranin O into a copolymer emulsion; the
development of a novel antimicrobial paint. RSC Advances, 5(33), 26364-26375.
Costa, T. R., Felisberto-Rodrigues, C., Meir, A., Prevost, M. S., Redzej, A., Trokter, M., &
Waksman, G. (2015). Secretion systems in Gram-negative bacteria: structural and
mechanistic insights. Nature Reviews Microbiology, 13(6), 343.
Danielson, N. B., Zaremba, J. D., Kaifosh, P., Bowler, J., Ladow, M., & Losonczy, A. (2016).
Sublayer-specific coding dynamics during spatial navigation and learning in hippocampal
area CA1. Neuron, 91(3), 652-665.
Flanagan, M., Sonnen, J. A., Keene, C. D., Hevner, R. F., & Montine, T. J. (2018). Molecular
Basis of Diseases of the Nervous System. In Molecular Pathology (Second Edition)(pp.
651-690).
García-Cabezas, M. Á., John, Y. J., Barbas, H., & Zikopoulos, B. (2016). The distinction of
neurons, glia and endothelial cells in the cerebral cortex: an algorithm based on
cytological features. Frontiers in neuroanatomy, 10, 107.

BIOLOGY 9
Ho, C. Y., Troncoso, J. C., Knox, D., Stark, W., & Eberhart, C. G. (2014). Beta‐Amyloid,
Phospho‐Tau and Alpha‐Synuclein Deposits Similar to Those in the Brain Are Not
Identified in the Eyes of Alzheimer's and Parkinson's Disease Patients. Brain
Pathology, 24(1), 25-32.
Merlini, M., Wanner, D., & Nitsch, R. M. (2016). Tau pathology-dependent remodeling of
cerebral arteries precedes Alzheimer's disease-related microvascular cerebral amyloid
angiopathy. Acta neuropathologica, 131(5), 737-752.
Saito, Y., Akazawa-Ogawa, Y., Matsumura, A., Saigoh, K., Itoh, S., Sutou, K., ... & Hara, Y.
(2016). Oxidation and interaction of DJ-1 with 20S proteasome in the erythrocytes of
early stage Parkinson’s disease patients. Scientific reports, 6, 30793.
Ho, C. Y., Troncoso, J. C., Knox, D., Stark, W., & Eberhart, C. G. (2014). Beta‐Amyloid,
Phospho‐Tau and Alpha‐Synuclein Deposits Similar to Those in the Brain Are Not
Identified in the Eyes of Alzheimer's and Parkinson's Disease Patients. Brain
Pathology, 24(1), 25-32.
Merlini, M., Wanner, D., & Nitsch, R. M. (2016). Tau pathology-dependent remodeling of
cerebral arteries precedes Alzheimer's disease-related microvascular cerebral amyloid
angiopathy. Acta neuropathologica, 131(5), 737-752.
Saito, Y., Akazawa-Ogawa, Y., Matsumura, A., Saigoh, K., Itoh, S., Sutou, K., ... & Hara, Y.
(2016). Oxidation and interaction of DJ-1 with 20S proteasome in the erythrocytes of
early stage Parkinson’s disease patients. Scientific reports, 6, 30793.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

