The Pharmaceutical Industry's Response to COVID-19: A Report
VerifiedAdded on 2022/02/04
|11
|2972
|37
Report
AI Summary
This report analyzes the extent to which R&D cost requirements, industry structure, and government subsidies affected businesses in the pharmaceutical industry in developing COVID-19 vaccines. It explores the impact of high fixed costs, clinical trials, and marketing expenses. The report examines the market structure, highlighting the oligopolistic nature of the industry, the role of patents, and the emergence of generic drugs. It also discusses the impact of government subsidies, including the provision of funding for R&D and bulk purchases, which shifted the supply curve and encouraged vaccine production. The report concludes by highlighting the importance of these factors in shaping the pharmaceutical industry's response to the pandemic and its ability to develop and distribute life-saving vaccines, particularly the use of mRNA vaccines. The report also touches upon evergreening and supplementary protection certificates as strategies used to maximize the economic value of innovations.

TO WHAT EXTENT HAVE THE
COST REQUIREMENTS OF
R&D, STRUCTURE OF THE
INDUSTRY AND GOV’T
SUBSIDY AFFECTED
BUSINESSES IN THE
PHARMACEUTICAL INDUSTRY
IN DEVELOPING VACCINES
FOR COVID-19?
Student ID: W18249
30th December, 2021
Notes to assessor
COST REQUIREMENTS OF
R&D, STRUCTURE OF THE
INDUSTRY AND GOV’T
SUBSIDY AFFECTED
BUSINESSES IN THE
PHARMACEUTICAL INDUSTRY
IN DEVELOPING VACCINES
FOR COVID-19?
Student ID: W18249
30th December, 2021
Notes to assessor
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Introduction
In terms of the extent of R&D cost requirements, industry structure, and government subsidy, and
how this has affected business in the pharmaceutical industry, I would say this has been very
impactful. This was especially evident when covid hit many geographical areas around the world.
For starters, the introduction of mRNA vaccines. This is said to have sped up the vaccine
development process, which is why covid-19 vaccines were able to carry out their purpose – to
protect people from dying as a result of this type of disease (Covid-19). Second, because of
government subsidies that encouraged vaccine production. Everything mentioned here has been
expanded upon and has resulted in this report.
(i) Cost Requirements of R&D
When it comes to cost requirements for any firm, the distinction between fixed and variable costs in
a regular firm and in the pharmaceutical industry context must be explained.
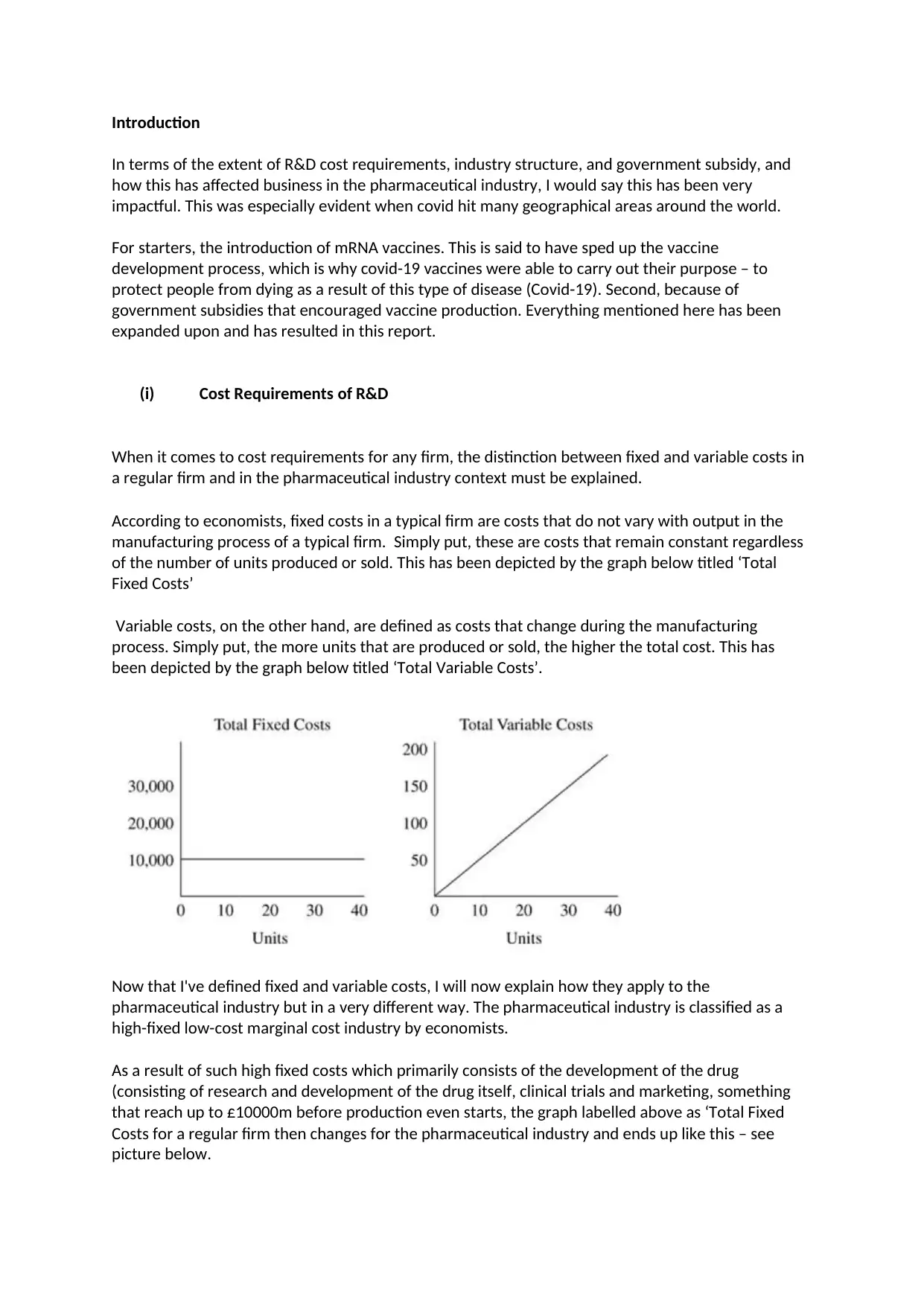
According to economists, fixed costs in a typical firm are costs that do not vary with output in the
manufacturing process of a typical firm. Simply put, these are costs that remain constant regardless
of the number of units produced or sold. This has been depicted by the graph below titled ‘Total
Fixed Costs’
Variable costs, on the other hand, are defined as costs that change during the manufacturing
process. Simply put, the more units that are produced or sold, the higher the total cost. This has
been depicted by the graph below titled ‘Total Variable Costs’.
Now that I've defined fixed and variable costs, I will now explain how they apply to the
pharmaceutical industry but in a very different way. The pharmaceutical industry is classified as a
high-fixed low-cost marginal cost industry by economists.
As a result of such high fixed costs which primarily consists of the development of the drug
(consisting of research and development of the drug itself, clinical trials and marketing, something
that reach up to £10000m before production even starts, the graph labelled above as ‘Total Fixed
Costs for a regular firm then changes for the pharmaceutical industry and ends up like this – see
picture below.
In terms of the extent of R&D cost requirements, industry structure, and government subsidy, and
how this has affected business in the pharmaceutical industry, I would say this has been very
impactful. This was especially evident when covid hit many geographical areas around the world.
For starters, the introduction of mRNA vaccines. This is said to have sped up the vaccine
development process, which is why covid-19 vaccines were able to carry out their purpose – to
protect people from dying as a result of this type of disease (Covid-19). Second, because of
government subsidies that encouraged vaccine production. Everything mentioned here has been
expanded upon and has resulted in this report.
(i) Cost Requirements of R&D
When it comes to cost requirements for any firm, the distinction between fixed and variable costs in
a regular firm and in the pharmaceutical industry context must be explained.
According to economists, fixed costs in a typical firm are costs that do not vary with output in the
manufacturing process of a typical firm. Simply put, these are costs that remain constant regardless
of the number of units produced or sold. This has been depicted by the graph below titled ‘Total
Fixed Costs’
Variable costs, on the other hand, are defined as costs that change during the manufacturing
process. Simply put, the more units that are produced or sold, the higher the total cost. This has
been depicted by the graph below titled ‘Total Variable Costs’.
Now that I've defined fixed and variable costs, I will now explain how they apply to the
pharmaceutical industry but in a very different way. The pharmaceutical industry is classified as a
high-fixed low-cost marginal cost industry by economists.
As a result of such high fixed costs which primarily consists of the development of the drug
(consisting of research and development of the drug itself, clinical trials and marketing, something
that reach up to £10000m before production even starts, the graph labelled above as ‘Total Fixed
Costs for a regular firm then changes for the pharmaceutical industry and ends up like this – see
picture below.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
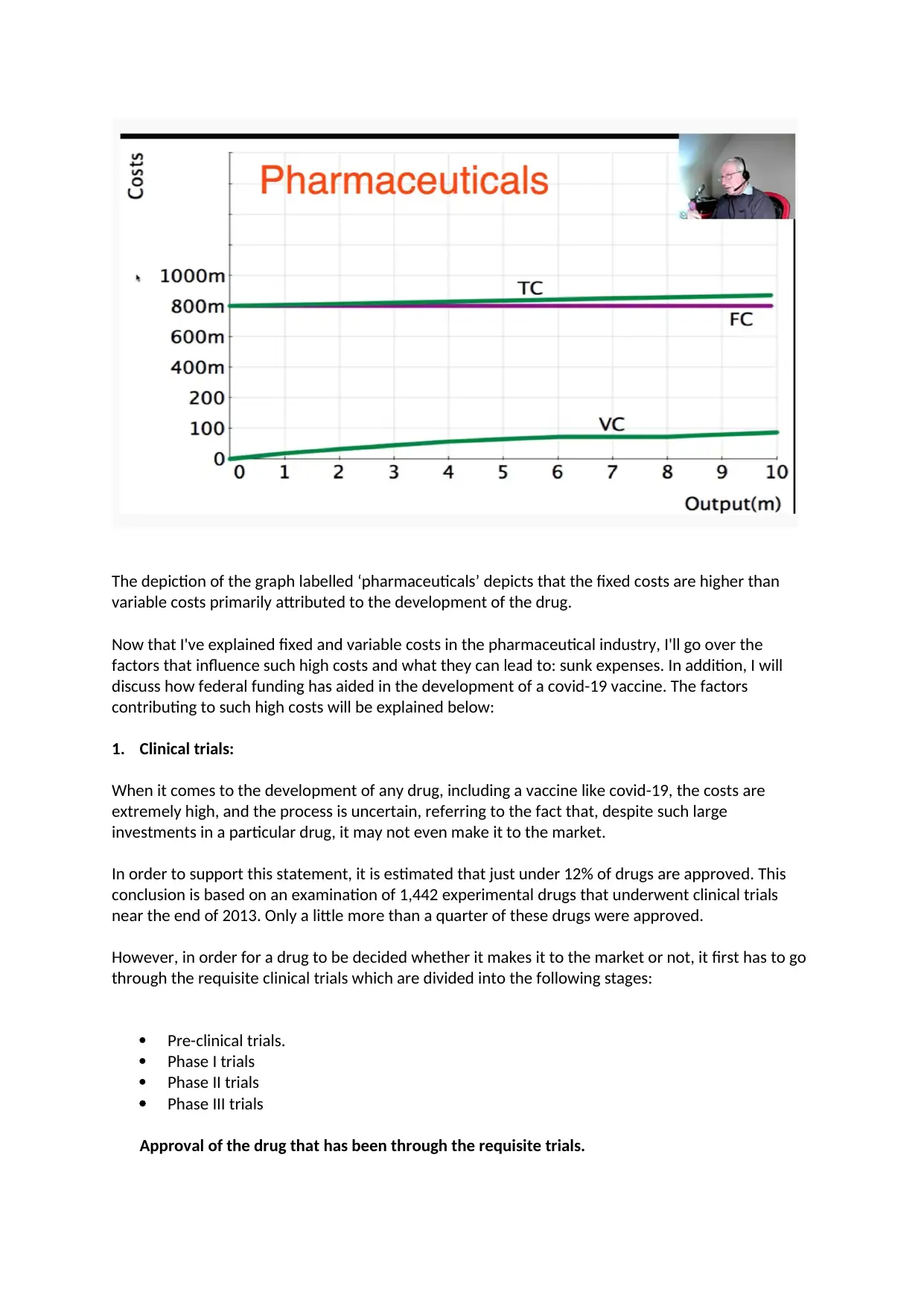
The depiction of the graph labelled ‘pharmaceuticals’ depicts that the fixed costs are higher than
variable costs primarily attributed to the development of the drug.
Now that I've explained fixed and variable costs in the pharmaceutical industry, I'll go over the
factors that influence such high costs and what they can lead to: sunk expenses. In addition, I will
discuss how federal funding has aided in the development of a covid-19 vaccine. The factors
contributing to such high costs will be explained below:
1. Clinical trials:
When it comes to the development of any drug, including a vaccine like covid-19, the costs are
extremely high, and the process is uncertain, referring to the fact that, despite such large
investments in a particular drug, it may not even make it to the market.
In order to support this statement, it is estimated that just under 12% of drugs are approved. This
conclusion is based on an examination of 1,442 experimental drugs that underwent clinical trials
near the end of 2013. Only a little more than a quarter of these drugs were approved.
However, in order for a drug to be decided whether it makes it to the market or not, it first has to go
through the requisite clinical trials which are divided into the following stages:
Pre-clinical trials.
Phase I trials
Phase II trials
Phase III trials
Approval of the drug that has been through the requisite trials.
variable costs primarily attributed to the development of the drug.
Now that I've explained fixed and variable costs in the pharmaceutical industry, I'll go over the
factors that influence such high costs and what they can lead to: sunk expenses. In addition, I will
discuss how federal funding has aided in the development of a covid-19 vaccine. The factors
contributing to such high costs will be explained below:
1. Clinical trials:
When it comes to the development of any drug, including a vaccine like covid-19, the costs are
extremely high, and the process is uncertain, referring to the fact that, despite such large
investments in a particular drug, it may not even make it to the market.
In order to support this statement, it is estimated that just under 12% of drugs are approved. This
conclusion is based on an examination of 1,442 experimental drugs that underwent clinical trials
near the end of 2013. Only a little more than a quarter of these drugs were approved.
However, in order for a drug to be decided whether it makes it to the market or not, it first has to go
through the requisite clinical trials which are divided into the following stages:
Pre-clinical trials.
Phase I trials
Phase II trials
Phase III trials
Approval of the drug that has been through the requisite trials.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
As mentioned above, despite such large investments when it comes to the development of any drug,
it may not even make it to the market. This can be known as sunk costs (expenses that are not
recoverable) in the pharmaceutical industry.
This is attributed to the fact that it must pass the three phases of clinical trials as well pass-through
regulatory agencies such as the Food and Drug Administration or the European Medicines Agency
which assess safety, efficacy, and quality in accordance with specific and detailed regulations.
Despite clinical trials taking 6-7 years, which includes all the phases above + regulatory review, for
covid-19, this was shortened attributed to the use of mRNA vaccines.
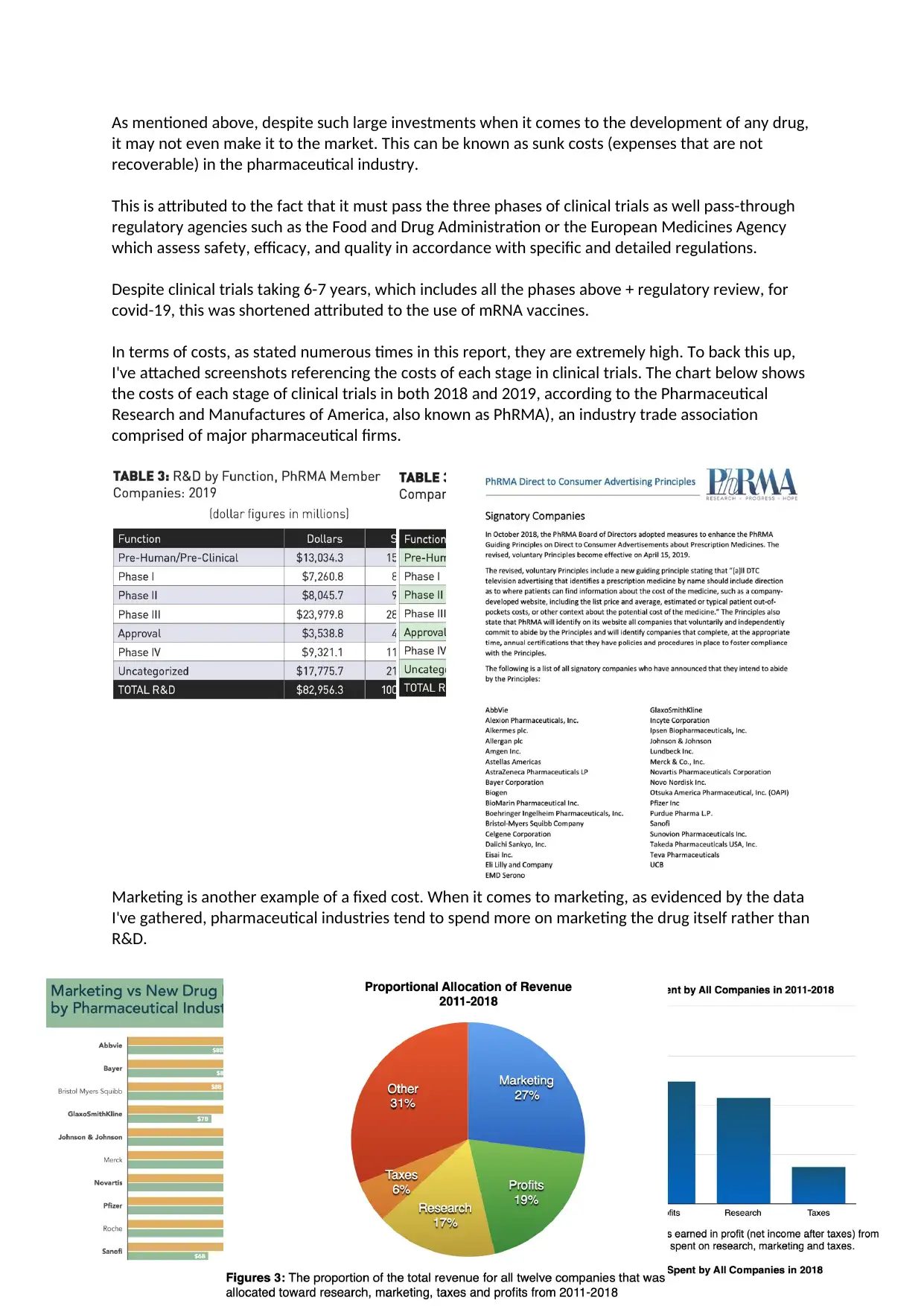
In terms of costs, as stated numerous times in this report, they are extremely high. To back this up,
I've attached screenshots referencing the costs of each stage in clinical trials. The chart below shows
the costs of each stage of clinical trials in both 2018 and 2019, according to the Pharmaceutical
Research and Manufactures of America, also known as PhRMA), an industry trade association
comprised of major pharmaceutical firms.
Marketing is another example of a fixed cost. When it comes to marketing, as evidenced by the data
I've gathered, pharmaceutical industries tend to spend more on marketing the drug itself rather than
R&D.
it may not even make it to the market. This can be known as sunk costs (expenses that are not
recoverable) in the pharmaceutical industry.
This is attributed to the fact that it must pass the three phases of clinical trials as well pass-through
regulatory agencies such as the Food and Drug Administration or the European Medicines Agency
which assess safety, efficacy, and quality in accordance with specific and detailed regulations.
Despite clinical trials taking 6-7 years, which includes all the phases above + regulatory review, for
covid-19, this was shortened attributed to the use of mRNA vaccines.
In terms of costs, as stated numerous times in this report, they are extremely high. To back this up,
I've attached screenshots referencing the costs of each stage in clinical trials. The chart below shows
the costs of each stage of clinical trials in both 2018 and 2019, according to the Pharmaceutical
Research and Manufactures of America, also known as PhRMA), an industry trade association
comprised of major pharmaceutical firms.
Marketing is another example of a fixed cost. When it comes to marketing, as evidenced by the data
I've gathered, pharmaceutical industries tend to spend more on marketing the drug itself rather than
R&D.
(ii) The Market Structure of the pharmaceutical industry
When it comes to the market structure of the pharmaceutical industry, this industry mainly consists
of a few large firms due to the large financial resources that are required to be successful and
because of the various barriers to entry such as high sunk cost which has discouraged many firms
from entering this industry.
As a result of only a few firms operating within this industry, one can argue that this industry is an
oligopoly which refers to the fact there are various firms operating in this industry. This conclusion is
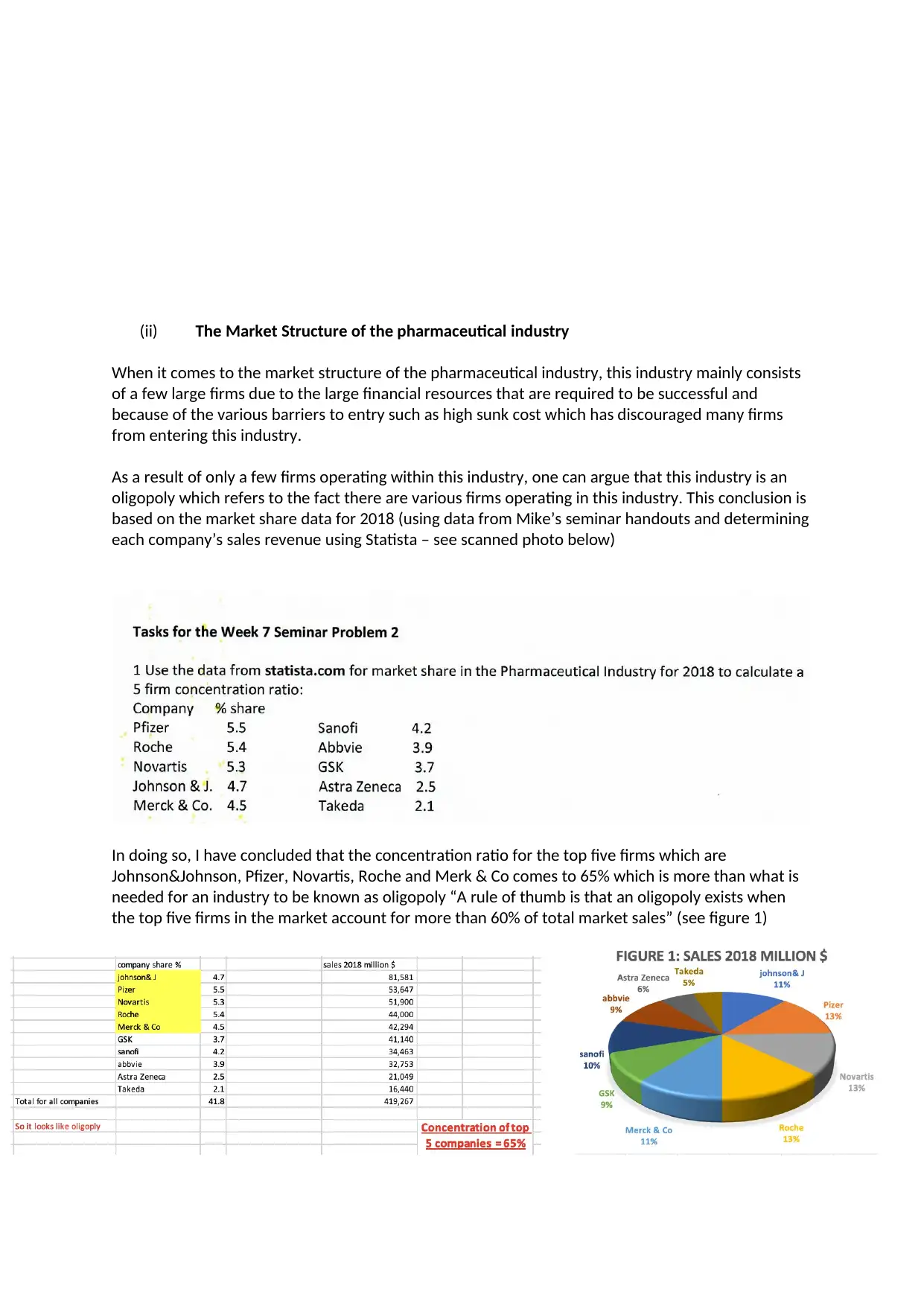
based on the market share data for 2018 (using data from Mike’s seminar handouts and determining
each company’s sales revenue using Statista – see scanned photo below)
In doing so, I have concluded that the concentration ratio for the top five firms which are
Johnson&Johnson, Pfizer, Novartis, Roche and Merk & Co comes to 65% which is more than what is
needed for an industry to be known as oligopoly “A rule of thumb is that an oligopoly exists when
the top five firms in the market account for more than 60% of total market sales” (see figure 1)
When it comes to the market structure of the pharmaceutical industry, this industry mainly consists
of a few large firms due to the large financial resources that are required to be successful and
because of the various barriers to entry such as high sunk cost which has discouraged many firms
from entering this industry.
As a result of only a few firms operating within this industry, one can argue that this industry is an
oligopoly which refers to the fact there are various firms operating in this industry. This conclusion is
based on the market share data for 2018 (using data from Mike’s seminar handouts and determining
each company’s sales revenue using Statista – see scanned photo below)
In doing so, I have concluded that the concentration ratio for the top five firms which are
Johnson&Johnson, Pfizer, Novartis, Roche and Merk & Co comes to 65% which is more than what is
needed for an industry to be known as oligopoly “A rule of thumb is that an oligopoly exists when
the top five firms in the market account for more than 60% of total market sales” (see figure 1)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Despite concluding that the market structure is that of an oligopoly, this is not entirely true due to
patents, which result in the aspect of monopoly arising. When a monopoly arises, two things
happen: evergreening and supplementary protection certificate, which are both inextricably linked
when it comes to a monopoly and will be explained below.
Patents are a type of legal protection that allows a company to keep other companies from
manufacturing their innovation for up to 20 years while earning significant profits from their
invention.
When a company applies for a patent, their intellectual property is protected, and competition is
stifled, allowing the company to retain exclusive rights to the creation of their innovation. When
they have exclusive rights, the company can (usually for a maximum of seven years) manufacture
their drug as well as sell it on the market.
As competition is stifled, the firm then gains market power because they can control and dictate
what prices they want to charge, allowing them to recoup their investments in the cost of research
and development and eventually earn a supernormal return on their investments. As a result of the
earning of supernormal return on their investments, firms can then use this to further their &D into
more R&D for new patents and drugs.
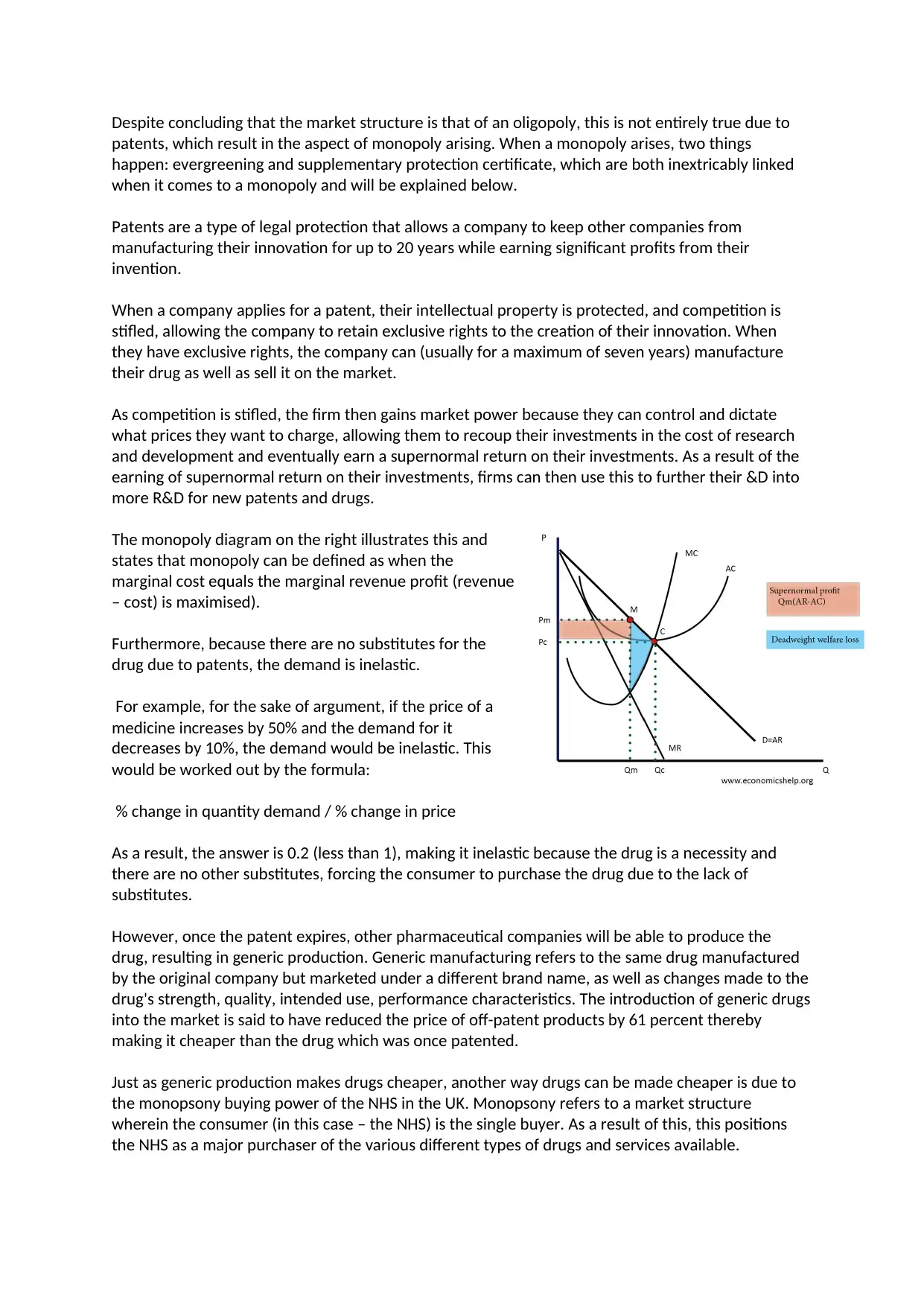
The monopoly diagram on the right illustrates this and
states that monopoly can be defined as when the
marginal cost equals the marginal revenue profit (revenue
– cost) is maximised).
Furthermore, because there are no substitutes for the
drug due to patents, the demand is inelastic.
For example, for the sake of argument, if the price of a
medicine increases by 50% and the demand for it
decreases by 10%, the demand would be inelastic. This
would be worked out by the formula:
% change in quantity demand / % change in price
As a result, the answer is 0.2 (less than 1), making it inelastic because the drug is a necessity and
there are no other substitutes, forcing the consumer to purchase the drug due to the lack of
substitutes.
However, once the patent expires, other pharmaceutical companies will be able to produce the
drug, resulting in generic production. Generic manufacturing refers to the same drug manufactured
by the original company but marketed under a different brand name, as well as changes made to the
drug's strength, quality, intended use, performance characteristics. The introduction of generic drugs
into the market is said to have reduced the price of off-patent products by 61 percent thereby
making it cheaper than the drug which was once patented.
Just as generic production makes drugs cheaper, another way drugs can be made cheaper is due to
the monopsony buying power of the NHS in the UK. Monopsony refers to a market structure
wherein the consumer (in this case – the NHS) is the single buyer. As a result of this, this positions
the NHS as a major purchaser of the various different types of drugs and services available.
patents, which result in the aspect of monopoly arising. When a monopoly arises, two things
happen: evergreening and supplementary protection certificate, which are both inextricably linked
when it comes to a monopoly and will be explained below.
Patents are a type of legal protection that allows a company to keep other companies from
manufacturing their innovation for up to 20 years while earning significant profits from their
invention.
When a company applies for a patent, their intellectual property is protected, and competition is
stifled, allowing the company to retain exclusive rights to the creation of their innovation. When
they have exclusive rights, the company can (usually for a maximum of seven years) manufacture
their drug as well as sell it on the market.
As competition is stifled, the firm then gains market power because they can control and dictate
what prices they want to charge, allowing them to recoup their investments in the cost of research
and development and eventually earn a supernormal return on their investments. As a result of the
earning of supernormal return on their investments, firms can then use this to further their &D into
more R&D for new patents and drugs.
The monopoly diagram on the right illustrates this and
states that monopoly can be defined as when the
marginal cost equals the marginal revenue profit (revenue
– cost) is maximised).
Furthermore, because there are no substitutes for the
drug due to patents, the demand is inelastic.
For example, for the sake of argument, if the price of a
medicine increases by 50% and the demand for it
decreases by 10%, the demand would be inelastic. This
would be worked out by the formula:
% change in quantity demand / % change in price
As a result, the answer is 0.2 (less than 1), making it inelastic because the drug is a necessity and
there are no other substitutes, forcing the consumer to purchase the drug due to the lack of
substitutes.
However, once the patent expires, other pharmaceutical companies will be able to produce the
drug, resulting in generic production. Generic manufacturing refers to the same drug manufactured
by the original company but marketed under a different brand name, as well as changes made to the
drug's strength, quality, intended use, performance characteristics. The introduction of generic drugs
into the market is said to have reduced the price of off-patent products by 61 percent thereby
making it cheaper than the drug which was once patented.
Just as generic production makes drugs cheaper, another way drugs can be made cheaper is due to
the monopsony buying power of the NHS in the UK. Monopsony refers to a market structure
wherein the consumer (in this case – the NHS) is the single buyer. As a result of this, this positions
the NHS as a major purchaser of the various different types of drugs and services available.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
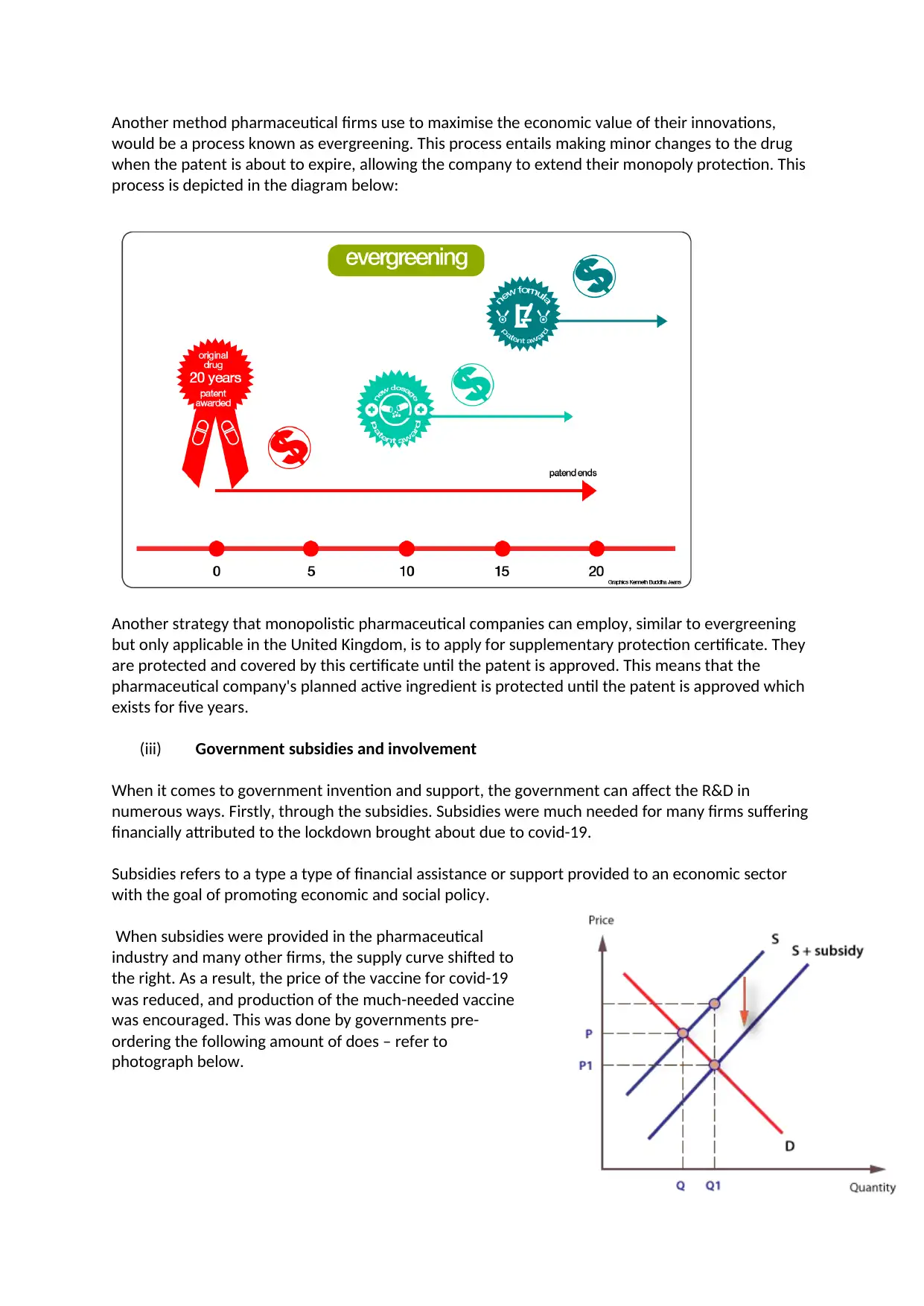
Another method pharmaceutical firms use to maximise the economic value of their innovations,
would be a process known as evergreening. This process entails making minor changes to the drug
when the patent is about to expire, allowing the company to extend their monopoly protection. This
process is depicted in the diagram below:
Another strategy that monopolistic pharmaceutical companies can employ, similar to evergreening
but only applicable in the United Kingdom, is to apply for supplementary protection certificate. They
are protected and covered by this certificate until the patent is approved. This means that the
pharmaceutical company's planned active ingredient is protected until the patent is approved which
exists for five years.
(iii) Government subsidies and involvement
When it comes to government invention and support, the government can affect the R&D in
numerous ways. Firstly, through the subsidies. Subsidies were much needed for many firms suffering
financially attributed to the lockdown brought about due to covid-19.
Subsidies refers to a type a type of financial assistance or support provided to an economic sector
with the goal of promoting economic and social policy.
When subsidies were provided in the pharmaceutical
industry and many other firms, the supply curve shifted to
the right. As a result, the price of the vaccine for covid-19
was reduced, and production of the much-needed vaccine
was encouraged. This was done by governments pre-
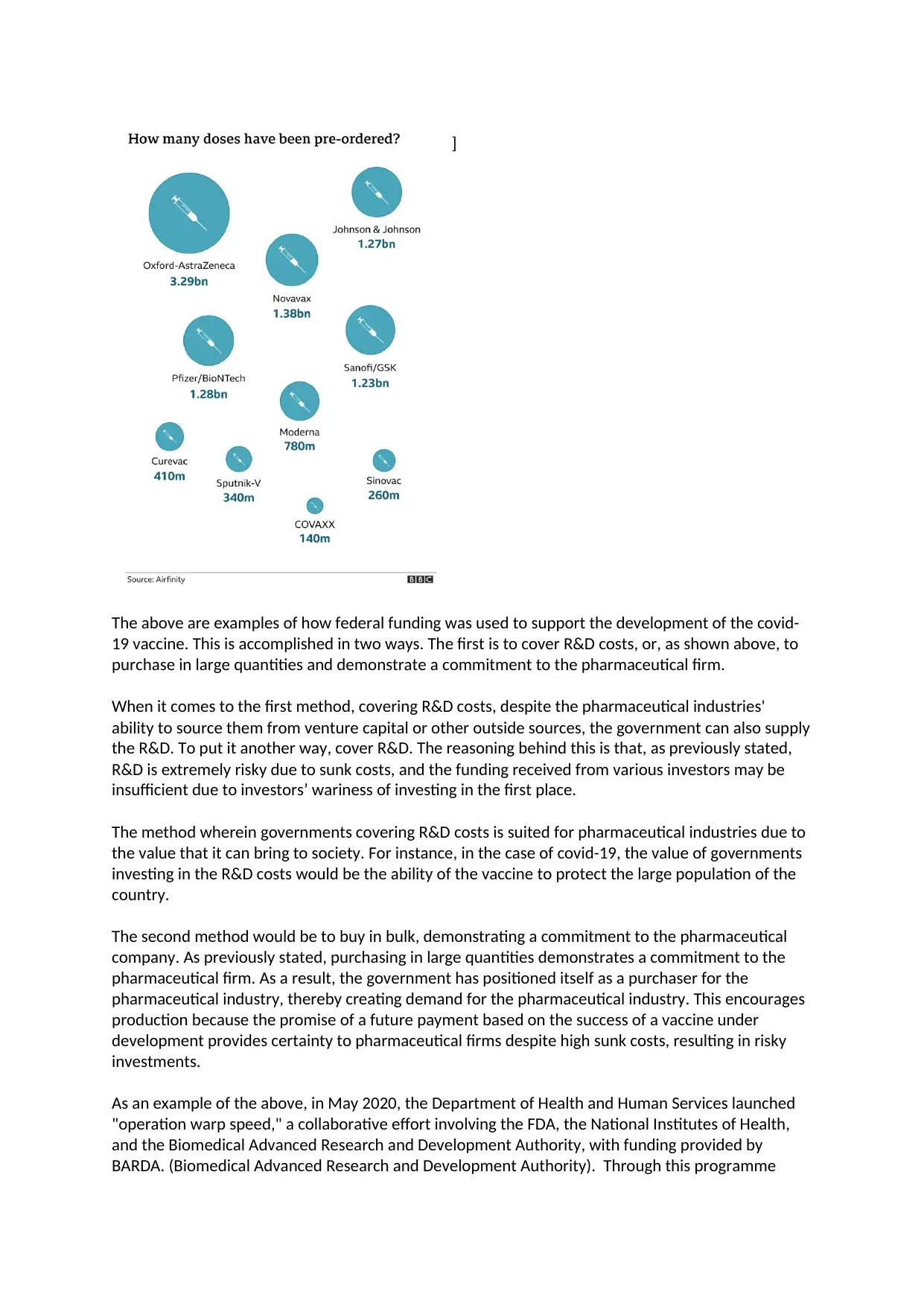
ordering the following amount of does – refer to
photograph below.
would be a process known as evergreening. This process entails making minor changes to the drug
when the patent is about to expire, allowing the company to extend their monopoly protection. This
process is depicted in the diagram below:
Another strategy that monopolistic pharmaceutical companies can employ, similar to evergreening
but only applicable in the United Kingdom, is to apply for supplementary protection certificate. They
are protected and covered by this certificate until the patent is approved. This means that the
pharmaceutical company's planned active ingredient is protected until the patent is approved which
exists for five years.
(iii) Government subsidies and involvement
When it comes to government invention and support, the government can affect the R&D in
numerous ways. Firstly, through the subsidies. Subsidies were much needed for many firms suffering
financially attributed to the lockdown brought about due to covid-19.
Subsidies refers to a type a type of financial assistance or support provided to an economic sector
with the goal of promoting economic and social policy.
When subsidies were provided in the pharmaceutical
industry and many other firms, the supply curve shifted to
the right. As a result, the price of the vaccine for covid-19
was reduced, and production of the much-needed vaccine
was encouraged. This was done by governments pre-
ordering the following amount of does – refer to
photograph below.
]
The above are examples of how federal funding was used to support the development of the covid-
19 vaccine. This is accomplished in two ways. The first is to cover R&D costs, or, as shown above, to
purchase in large quantities and demonstrate a commitment to the pharmaceutical firm.
When it comes to the first method, covering R&D costs, despite the pharmaceutical industries'
ability to source them from venture capital or other outside sources, the government can also supply
the R&D. To put it another way, cover R&D. The reasoning behind this is that, as previously stated,
R&D is extremely risky due to sunk costs, and the funding received from various investors may be
insufficient due to investors’ wariness of investing in the first place.
The method wherein governments covering R&D costs is suited for pharmaceutical industries due to
the value that it can bring to society. For instance, in the case of covid-19, the value of governments
investing in the R&D costs would be the ability of the vaccine to protect the large population of the
country.
The second method would be to buy in bulk, demonstrating a commitment to the pharmaceutical
company. As previously stated, purchasing in large quantities demonstrates a commitment to the
pharmaceutical firm. As a result, the government has positioned itself as a purchaser for the
pharmaceutical industry, thereby creating demand for the pharmaceutical industry. This encourages
production because the promise of a future payment based on the success of a vaccine under
development provides certainty to pharmaceutical firms despite high sunk costs, resulting in risky
investments.
As an example of the above, in May 2020, the Department of Health and Human Services launched
"operation warp speed," a collaborative effort involving the FDA, the National Institutes of Health,
and the Biomedical Advanced Research and Development Authority, with funding provided by
BARDA. (Biomedical Advanced Research and Development Authority). Through this programme
The above are examples of how federal funding was used to support the development of the covid-
19 vaccine. This is accomplished in two ways. The first is to cover R&D costs, or, as shown above, to
purchase in large quantities and demonstrate a commitment to the pharmaceutical firm.
When it comes to the first method, covering R&D costs, despite the pharmaceutical industries'
ability to source them from venture capital or other outside sources, the government can also supply
the R&D. To put it another way, cover R&D. The reasoning behind this is that, as previously stated,
R&D is extremely risky due to sunk costs, and the funding received from various investors may be
insufficient due to investors’ wariness of investing in the first place.
The method wherein governments covering R&D costs is suited for pharmaceutical industries due to
the value that it can bring to society. For instance, in the case of covid-19, the value of governments
investing in the R&D costs would be the ability of the vaccine to protect the large population of the
country.
The second method would be to buy in bulk, demonstrating a commitment to the pharmaceutical
company. As previously stated, purchasing in large quantities demonstrates a commitment to the
pharmaceutical firm. As a result, the government has positioned itself as a purchaser for the
pharmaceutical industry, thereby creating demand for the pharmaceutical industry. This encourages
production because the promise of a future payment based on the success of a vaccine under
development provides certainty to pharmaceutical firms despite high sunk costs, resulting in risky
investments.
As an example of the above, in May 2020, the Department of Health and Human Services launched
"operation warp speed," a collaborative effort involving the FDA, the National Institutes of Health,
and the Biomedical Advanced Research and Development Authority, with funding provided by
BARDA. (Biomedical Advanced Research and Development Authority). Through this programme
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

codenamed “Operation Warp Speed”, The government provided over $19 billion to help seven
pharmaceutical companies develop and manufacture the covid-19 vaccine. As a result, out of the 7
companies, 5 accepted the government’s financial assistance which enabled them to ramp up their
production capabilities.
In conclusion, when it comes to R&D cost requirements, industry structure, and government subsidy,
I would say that there has always been a high fixed in this industry due to the nature of the industry,
i.e. risky investment in the development of a drug or, more specifically, in the development of the
covid vaccine. As a result of such high costs, governments have invented (compelled to do so by
covid), resulting in dramatic cost reductions. Furthermore, because large sums of money have been
poured into this industry to assist pharmaceutical firms, the speed of development has been
accelerated through the ramping up of production facilities as well as the introduction of new
technology to accelerate the development of the covid-19 vaccine.
References:
Policymed.com. 2022. [online] Available at: <https://www.policymed.com/2014/12/a-
tough-road-cost-to-develop-one-new-drug-is-26-billion-approval-rate-for-drugs-entering-
clinical-de.html> [Accessed 8 January 2022].
Truecostofhealthcare.org. 2022. [online] Available at:
<https://truecostofhealthcare.org/wp-content/uploads/2019/03/Totals-for-All.pdf>
[Accessed 11 January 2022].
Statista. 2022. Novartis revenues 2007-2020 | Statista. [online] Available at:
<https://www.statista.com/statistics/264238/novartis-revenues-since-2007/>
[Accessed 6 December 2021].
Statista. 2022. Roche Group total sales 2006-2020 | Statista. [online] Available at:
<https://www.statista.com/statistics/266517/roche-group-sales-since-2006/>
[Accessed 5 January 2021].
Statista. 2022. Johnson & Johnson total sales 2005-2020 | Statista. [online] Available
at: <https://www.statista.com/statistics/266403/total-revenue-of-johnson-und-
johnson-since-2004/> [Accessed 11 November 2021].
Statista. 2022. Merck and Co revenue 2006-2020 | Statista. [online] Available at:
<https://www.statista.com/statistics/272350/revenue-of-merck-and-co/> [Accessed 7
December 2021].
Statista. 2022. Sanofi revenue 2006-2020 | Statista. [online] Available at:
<https://www.statista.com/statistics/266509/revenue-of-sanofi-since-2006/>
[Accessed 2 December 2021].
Statista. 2022. AbbVie revenue 2010-2020 | Statista. [online] Available at:
<https://www.statista.com/statistics/417030/revenue-of-abbvie/> [Accessed 1
December 2021].
AstraZeneca revenue 2006-2020 | Statista (2022). Statista. Available from
https://www.statista.com/statistics/266542/revenue-of-astrazeneca-since-2006/
[Accessed 1 October 2021].
pharmaceutical companies develop and manufacture the covid-19 vaccine. As a result, out of the 7
companies, 5 accepted the government’s financial assistance which enabled them to ramp up their
production capabilities.
In conclusion, when it comes to R&D cost requirements, industry structure, and government subsidy,
I would say that there has always been a high fixed in this industry due to the nature of the industry,
i.e. risky investment in the development of a drug or, more specifically, in the development of the
covid vaccine. As a result of such high costs, governments have invented (compelled to do so by
covid), resulting in dramatic cost reductions. Furthermore, because large sums of money have been
poured into this industry to assist pharmaceutical firms, the speed of development has been
accelerated through the ramping up of production facilities as well as the introduction of new
technology to accelerate the development of the covid-19 vaccine.
References:
Policymed.com. 2022. [online] Available at: <https://www.policymed.com/2014/12/a-
tough-road-cost-to-develop-one-new-drug-is-26-billion-approval-rate-for-drugs-entering-
clinical-de.html> [Accessed 8 January 2022].
Truecostofhealthcare.org. 2022. [online] Available at:
<https://truecostofhealthcare.org/wp-content/uploads/2019/03/Totals-for-All.pdf>
[Accessed 11 January 2022].
Statista. 2022. Novartis revenues 2007-2020 | Statista. [online] Available at:
<https://www.statista.com/statistics/264238/novartis-revenues-since-2007/>
[Accessed 6 December 2021].
Statista. 2022. Roche Group total sales 2006-2020 | Statista. [online] Available at:
<https://www.statista.com/statistics/266517/roche-group-sales-since-2006/>
[Accessed 5 January 2021].
Statista. 2022. Johnson & Johnson total sales 2005-2020 | Statista. [online] Available
at: <https://www.statista.com/statistics/266403/total-revenue-of-johnson-und-
johnson-since-2004/> [Accessed 11 November 2021].
Statista. 2022. Merck and Co revenue 2006-2020 | Statista. [online] Available at:
<https://www.statista.com/statistics/272350/revenue-of-merck-and-co/> [Accessed 7
December 2021].
Statista. 2022. Sanofi revenue 2006-2020 | Statista. [online] Available at:
<https://www.statista.com/statistics/266509/revenue-of-sanofi-since-2006/>
[Accessed 2 December 2021].
Statista. 2022. AbbVie revenue 2010-2020 | Statista. [online] Available at:
<https://www.statista.com/statistics/417030/revenue-of-abbvie/> [Accessed 1
December 2021].
AstraZeneca revenue 2006-2020 | Statista (2022). Statista. Available from
https://www.statista.com/statistics/266542/revenue-of-astrazeneca-since-2006/
[Accessed 1 October 2021].
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Coronavirus and homeworking in the UK - Office for National Statistics
(2022). Ons.gov.uk. Available from
https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentande
mployeetypes/bulletins/coronavirusandhomeworkingintheuk/april2020 [Accessed 4
November 2021].
Coronavirus and homeworking in the UK - Office for National Statistics
(2022). Ons.gov.uk. Available from
https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentande
mployeetypes/bulletins/coronavirusandhomeworkingintheuk/april2020 [Accessed 11
January 2022].
Data reveals damage done to UK economy by Covid-19 (2022). Ft.com. Available
from https://www.ft.com/content/1602e772-ea55-4c83-ad1a-ddba2ce38fc7 [Accessed
6 December 2021].
Ahip.org. Available from https://www.ahip.org/wp-content/uploads/202107-AHIP_Rx-
MarketingRD-1.pdf [Accessed 11 January 2022].
New Study: In the Midst of COVID-19 Crisis, 7 out of 10 Big Pharma Companies Spent
More on Sales and Marketing than R&D - AHIP (2022). AHIP. Available from
https://www.ahip.org/new-study-in-the-midst-of-covid-19-crisis-7-out-of-10-big-
pharma-companies-spent-more-on-sales-and-marketing-than-rd [Accessed 3 January
2021].
PhRMA. (n.d.). https://phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/P-R/
PhRMA_Membership_Survey_2020.pdf.
https://www.phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/P-R/
PhRMA_2019_membership_survey_Final.pdf
https://www.investopedia.com/terms/c/concentrationratio.asp
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2630351/
Notes from lessons/lectures
(2022). Ons.gov.uk. Available from
https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentande
mployeetypes/bulletins/coronavirusandhomeworkingintheuk/april2020 [Accessed 4
November 2021].
Coronavirus and homeworking in the UK - Office for National Statistics
(2022). Ons.gov.uk. Available from
https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentande
mployeetypes/bulletins/coronavirusandhomeworkingintheuk/april2020 [Accessed 11
January 2022].
Data reveals damage done to UK economy by Covid-19 (2022). Ft.com. Available
from https://www.ft.com/content/1602e772-ea55-4c83-ad1a-ddba2ce38fc7 [Accessed
6 December 2021].
Ahip.org. Available from https://www.ahip.org/wp-content/uploads/202107-AHIP_Rx-
MarketingRD-1.pdf [Accessed 11 January 2022].
New Study: In the Midst of COVID-19 Crisis, 7 out of 10 Big Pharma Companies Spent
More on Sales and Marketing than R&D - AHIP (2022). AHIP. Available from
https://www.ahip.org/new-study-in-the-midst-of-covid-19-crisis-7-out-of-10-big-
pharma-companies-spent-more-on-sales-and-marketing-than-rd [Accessed 3 January
2021].
PhRMA. (n.d.). https://phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/P-R/
PhRMA_Membership_Survey_2020.pdf.
https://www.phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/P-R/
PhRMA_2019_membership_survey_Final.pdf
https://www.investopedia.com/terms/c/concentrationratio.asp
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2630351/
Notes from lessons/lectures
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


