Access to Higher Education Diploma Chemistry Research: Planning a Practical Investigation (Chemistry)
VerifiedAdded on 2023/05/30
|16
|3213
|346
AI Summary
This is a research project template for Access to Higher Education Diploma Chemistry Research: Planning a Practical Investigation (Chemistry). It includes a student declaration, learning outcomes and assessment criteria, proposed plan for the investigation, methodology, and risk assessment.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

“DistanceLearningCentre.com
STUDENT ASSESSMENT ANSWER SHEET
COURSE: Access to Higher Education Diploma
SUBJECT: Chemistry
UNIT TITLE: Research: Planning a Practical Investigation (Chemistry)
LEVEL: 3 (Graded)
CREDITS: 3
PART 1: Student declaration
Please complete all relevant information below:
I understand that copying / taking ideas from other sources (e.g. reference books, journals,
internet, and tutor hand-outs) without acknowledging them is plagiarism.
I confirm that:
This assignment is all my own work.
All contributions taken from other reading and research have been referenced
accurately.
Any direct quotations taken from other reading and research have been acknowledged
and attributed accurately.
I have attached a bibliography listing all sources used in producing this assignment.
I have added the word count below. (Note: your work must be within the word count
range: for a 3 credit unit this is 1000-1500 words, and for a 6 credit unit it is 2000-2500
words.)
I have read and understood the Ascentis - Malpractice and Maladministration
Policy, and understand the consequences of non-compliance with this document.
Your full name: Your signature *:
Date on which
assessment was set:
Date due: Date submitted: Extension - date due
(if applicable):
Actual word count per
TAQ:
Remember that your assessment’s overall word count should
sit between 1000-1500 (3 credit unit) and 2000-2500 (6 credit
unit)
* Please type your name into the signature box above and upload this document to your
Learner Account. This will be accepted as your electronic signature.
© DistanceLearningCentre.com, 2017
1
STUDENT ASSESSMENT ANSWER SHEET
COURSE: Access to Higher Education Diploma
SUBJECT: Chemistry
UNIT TITLE: Research: Planning a Practical Investigation (Chemistry)
LEVEL: 3 (Graded)
CREDITS: 3
PART 1: Student declaration
Please complete all relevant information below:
I understand that copying / taking ideas from other sources (e.g. reference books, journals,
internet, and tutor hand-outs) without acknowledging them is plagiarism.
I confirm that:
This assignment is all my own work.
All contributions taken from other reading and research have been referenced
accurately.
Any direct quotations taken from other reading and research have been acknowledged
and attributed accurately.
I have attached a bibliography listing all sources used in producing this assignment.
I have added the word count below. (Note: your work must be within the word count
range: for a 3 credit unit this is 1000-1500 words, and for a 6 credit unit it is 2000-2500
words.)
I have read and understood the Ascentis - Malpractice and Maladministration
Policy, and understand the consequences of non-compliance with this document.
Your full name: Your signature *:
Date on which
assessment was set:
Date due: Date submitted: Extension - date due
(if applicable):
Actual word count per
TAQ:
Remember that your assessment’s overall word count should
sit between 1000-1500 (3 credit unit) and 2000-2500 (6 credit
unit)
* Please type your name into the signature box above and upload this document to your
Learner Account. This will be accepted as your electronic signature.
© DistanceLearningCentre.com, 2017
1
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
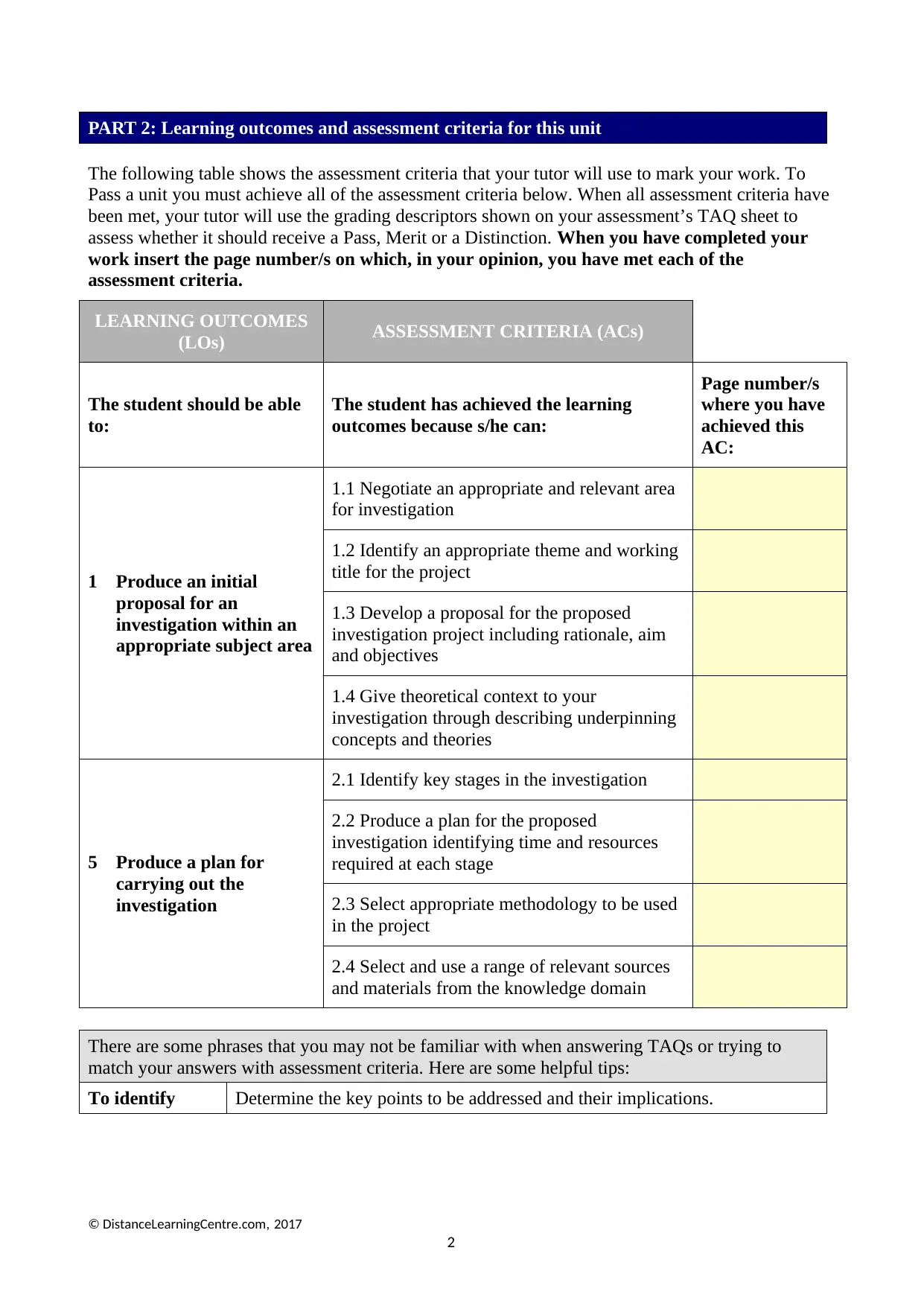
PART 2: Learning outcomes and assessment criteria for this unit
The following table shows the assessment criteria that your tutor will use to mark your work. To
Pass a unit you must achieve all of the assessment criteria below. When all assessment criteria have
been met, your tutor will use the grading descriptors shown on your assessment’s TAQ sheet to
assess whether it should receive a Pass, Merit or a Distinction. When you have completed your
work insert the page number/s on which, in your opinion, you have met each of the
assessment criteria.
LEARNING OUTCOMES
(LOs) ASSESSMENT CRITERIA (ACs)
The student should be able
to:
The student has achieved the learning
outcomes because s/he can:
Page number/s
where you have
achieved this
AC:
1 Produce an initial
proposal for an
investigation within an
appropriate subject area
1.1 Negotiate an appropriate and relevant area
for investigation
1.2 Identify an appropriate theme and working
title for the project
1.3 Develop a proposal for the proposed
investigation project including rationale, aim
and objectives
1.4 Give theoretical context to your
investigation through describing underpinning
concepts and theories
5 Produce a plan for
carrying out the
investigation
2.1 Identify key stages in the investigation
2.2 Produce a plan for the proposed
investigation identifying time and resources
required at each stage
2.3 Select appropriate methodology to be used
in the project
2.4 Select and use a range of relevant sources
and materials from the knowledge domain
There are some phrases that you may not be familiar with when answering TAQs or trying to
match your answers with assessment criteria. Here are some helpful tips:
To identify Determine the key points to be addressed and their implications.
© DistanceLearningCentre.com, 2017
2
The following table shows the assessment criteria that your tutor will use to mark your work. To
Pass a unit you must achieve all of the assessment criteria below. When all assessment criteria have
been met, your tutor will use the grading descriptors shown on your assessment’s TAQ sheet to
assess whether it should receive a Pass, Merit or a Distinction. When you have completed your
work insert the page number/s on which, in your opinion, you have met each of the
assessment criteria.
LEARNING OUTCOMES
(LOs) ASSESSMENT CRITERIA (ACs)
The student should be able
to:
The student has achieved the learning
outcomes because s/he can:
Page number/s
where you have
achieved this
AC:
1 Produce an initial
proposal for an
investigation within an
appropriate subject area
1.1 Negotiate an appropriate and relevant area
for investigation
1.2 Identify an appropriate theme and working
title for the project
1.3 Develop a proposal for the proposed
investigation project including rationale, aim
and objectives
1.4 Give theoretical context to your
investigation through describing underpinning
concepts and theories
5 Produce a plan for
carrying out the
investigation
2.1 Identify key stages in the investigation
2.2 Produce a plan for the proposed
investigation identifying time and resources
required at each stage
2.3 Select appropriate methodology to be used
in the project
2.4 Select and use a range of relevant sources
and materials from the knowledge domain
There are some phrases that you may not be familiar with when answering TAQs or trying to
match your answers with assessment criteria. Here are some helpful tips:
To identify Determine the key points to be addressed and their implications.
© DistanceLearningCentre.com, 2017
2

Further resources:
You will need to be logged in to your Learner Account to access these
resources.
We advise that you check the Ascentis Subject Set Unit
Specifications – Chemistry for the ‘indicative content’ of the
unit, as this may help you to understand how you could meet
specific assessment criteria.
DLC Student Handbook
DLC
Librar
y:
Log in to your Learner Account and click on ‘Library’ to view
various resources to help you with your learning.
For any table, you can add more rows if necessary. If you are unsure
about how to do this, please see the following:
DLC Learning materials: Study Skills - How to use I.T.
PART 3: Your comments on this assignment”
“
© DistanceLearningCentre.com, 2017
3
You will need to be logged in to your Learner Account to access these
resources.
We advise that you check the Ascentis Subject Set Unit
Specifications – Chemistry for the ‘indicative content’ of the
unit, as this may help you to understand how you could meet
specific assessment criteria.
DLC Student Handbook
DLC
Librar
y:
Log in to your Learner Account and click on ‘Library’ to view
various resources to help you with your learning.
For any table, you can add more rows if necessary. If you are unsure
about how to do this, please see the following:
DLC Learning materials: Study Skills - How to use I.T.
PART 3: Your comments on this assignment”
“
© DistanceLearningCentre.com, 2017
3
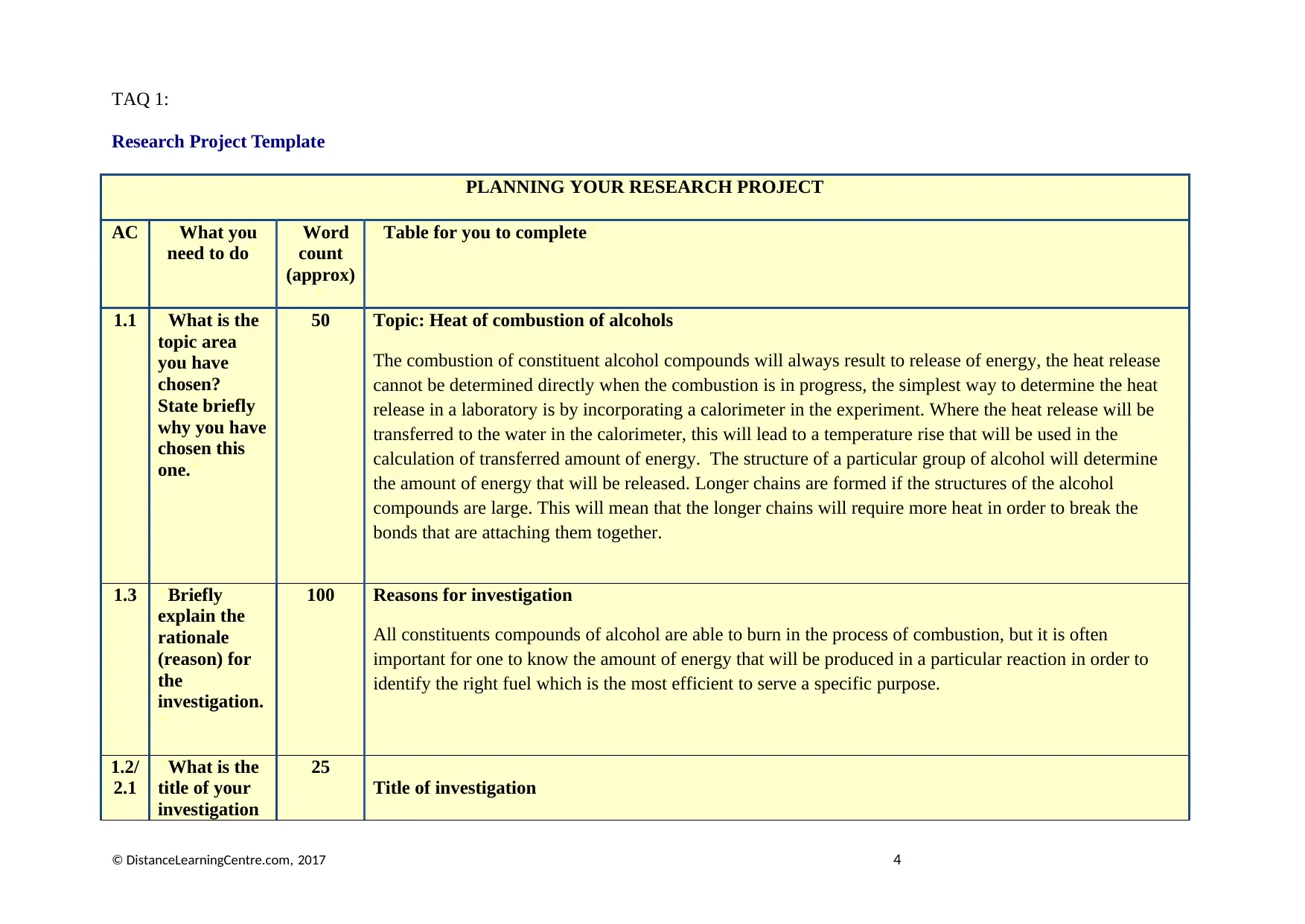
TAQ 1:
Research Project Template
"PLANNING YOUR RESEARCH PROJECT"
AC "What you
need to do"
"Word
count
(approx)
"
"Table for you to complete"
1.1 "What is the
topic area
you have
chosen?
State briefly
why you have
chosen this
one."
50 Topic: Heat of combustion of alcohols
The combustion of constituent alcohol compounds will always result to release of energy, the heat release
cannot be determined directly when the combustion is in progress, the simplest way to determine the heat
release in a laboratory is by incorporating a calorimeter in the experiment. Where the heat release will be
transferred to the water in the calorimeter, this will lead to a temperature rise that will be used in the
calculation of transferred amount of energy. The structure of a particular group of alcohol will determine
the amount of energy that will be released. Longer chains are formed if the structures of the alcohol
compounds are large. This will mean that the longer chains will require more heat in order to break the
bonds that are attaching them together.
1.3 "Briefly
explain the
rationale
(reason) for
the
investigation.
"
100 Reasons for investigation
All constituents compounds of alcohol are able to burn in the process of combustion, but it is often
important for one to know the amount of energy that will be produced in a particular reaction in order to
identify the right fuel which is the most efficient to serve a specific purpose.
1.2/
2.1
"What is the
title of your
investigation
25
Title of investigation
© DistanceLearningCentre.com, 2017 4
Research Project Template
"PLANNING YOUR RESEARCH PROJECT"
AC "What you
need to do"
"Word
count
(approx)
"
"Table for you to complete"
1.1 "What is the
topic area
you have
chosen?
State briefly
why you have
chosen this
one."
50 Topic: Heat of combustion of alcohols
The combustion of constituent alcohol compounds will always result to release of energy, the heat release
cannot be determined directly when the combustion is in progress, the simplest way to determine the heat
release in a laboratory is by incorporating a calorimeter in the experiment. Where the heat release will be
transferred to the water in the calorimeter, this will lead to a temperature rise that will be used in the
calculation of transferred amount of energy. The structure of a particular group of alcohol will determine
the amount of energy that will be released. Longer chains are formed if the structures of the alcohol
compounds are large. This will mean that the longer chains will require more heat in order to break the
bonds that are attaching them together.
1.3 "Briefly
explain the
rationale
(reason) for
the
investigation.
"
100 Reasons for investigation
All constituents compounds of alcohol are able to burn in the process of combustion, but it is often
important for one to know the amount of energy that will be produced in a particular reaction in order to
identify the right fuel which is the most efficient to serve a specific purpose.
1.2/
2.1
"What is the
title of your
investigation
25
Title of investigation
© DistanceLearningCentre.com, 2017 4
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
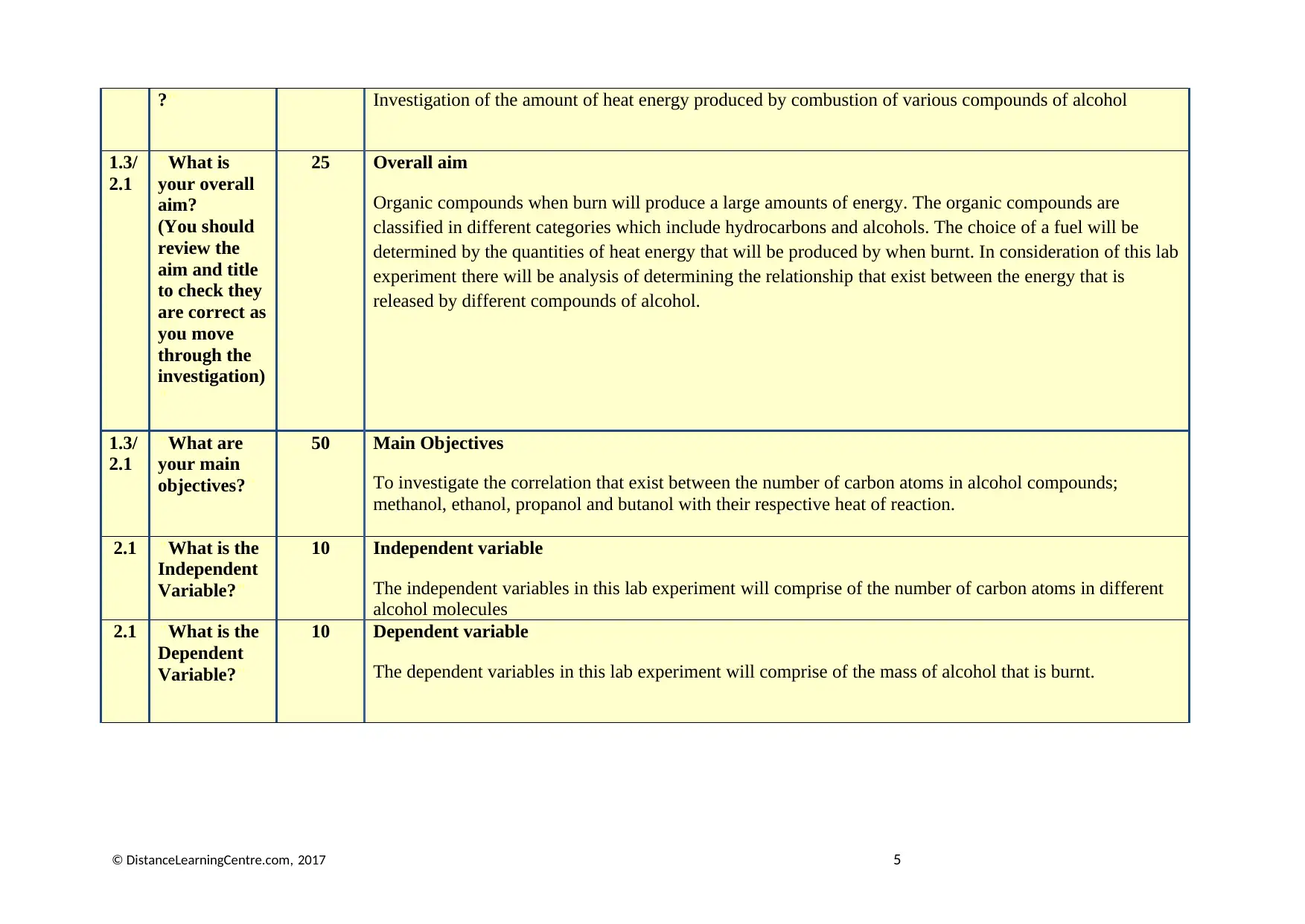
?" Investigation of the amount of heat energy produced by combustion of various compounds of alcohol
1.3/
2.1
"What is
your overall
aim?
(You should
review the
aim and title
to check they
are correct as
you move
through the
investigation)
"
25 Overall aim
Organic compounds when burn will produce a large amounts of energy. The organic compounds are
classified in different categories which include hydrocarbons and alcohols. The choice of a fuel will be
determined by the quantities of heat energy that will be produced by when burnt. In consideration of this lab
experiment there will be analysis of determining the relationship that exist between the energy that is
released by different compounds of alcohol.
1.3/
2.1
"What are
your main
objectives?"
50 Main Objectives
To investigate the correlation that exist between the number of carbon atoms in alcohol compounds;
methanol, ethanol, propanol and butanol with their respective heat of reaction.
2.1 "What is the
Independent
Variable?"
10 Independent variable
The independent variables in this lab experiment will comprise of the number of carbon atoms in different
alcohol molecules
2.1 "What is the
Dependent
Variable?"
10 Dependent variable
The dependent variables in this lab experiment will comprise of the mass of alcohol that is burnt.
© DistanceLearningCentre.com, 2017 5
1.3/
2.1
"What is
your overall
aim?
(You should
review the
aim and title
to check they
are correct as
you move
through the
investigation)
"
25 Overall aim
Organic compounds when burn will produce a large amounts of energy. The organic compounds are
classified in different categories which include hydrocarbons and alcohols. The choice of a fuel will be
determined by the quantities of heat energy that will be produced by when burnt. In consideration of this lab
experiment there will be analysis of determining the relationship that exist between the energy that is
released by different compounds of alcohol.
1.3/
2.1
"What are
your main
objectives?"
50 Main Objectives
To investigate the correlation that exist between the number of carbon atoms in alcohol compounds;
methanol, ethanol, propanol and butanol with their respective heat of reaction.
2.1 "What is the
Independent
Variable?"
10 Independent variable
The independent variables in this lab experiment will comprise of the number of carbon atoms in different
alcohol molecules
2.1 "What is the
Dependent
Variable?"
10 Dependent variable
The dependent variables in this lab experiment will comprise of the mass of alcohol that is burnt.
© DistanceLearningCentre.com, 2017 5

"INTRODUCTION (1.4)"
"This shows your understanding of the science behind the experiment but it must be relevant. In the introduction you can include any
background information you have found whilst researching your topic.
(Include in-text referencing in your answer)"
500 words
The compound of alcohol contains hydrogen, oxygen and carbon; they are classified to members of hydrocarbons that contain a functional
group of –OH. One of the characteristic of alcohol is they are homologous series having a function group –OH, which differentiates it from
other members of hydrocarbons; the function group will influence the nature of how alcohol compounds will react (Hellier, Ladommatos, Allan
and Rogerson, 2012).
Alcohol is represented in a general formula of CnH2n+1OH, where n represent the total number of carbon present in a particular alcohol. The first
alcohol group has one carbon and is known as methanol, its molecular formula is represented as CH3OH. As one move down this homologous
series the number of carbon will too be increasing. The molecules of alcohol will differ from each subsequent molecule by
-CH2, this can be represented in the example of methanol and ethanol respectively on figure 1.
© DistanceLearningCentre.com, 2017 6
"This shows your understanding of the science behind the experiment but it must be relevant. In the introduction you can include any
background information you have found whilst researching your topic.
(Include in-text referencing in your answer)"
500 words
The compound of alcohol contains hydrogen, oxygen and carbon; they are classified to members of hydrocarbons that contain a functional
group of –OH. One of the characteristic of alcohol is they are homologous series having a function group –OH, which differentiates it from
other members of hydrocarbons; the function group will influence the nature of how alcohol compounds will react (Hellier, Ladommatos, Allan
and Rogerson, 2012).
Alcohol is represented in a general formula of CnH2n+1OH, where n represent the total number of carbon present in a particular alcohol. The first
alcohol group has one carbon and is known as methanol, its molecular formula is represented as CH3OH. As one move down this homologous
series the number of carbon will too be increasing. The molecules of alcohol will differ from each subsequent molecule by
-CH2, this can be represented in the example of methanol and ethanol respectively on figure 1.
© DistanceLearningCentre.com, 2017 6
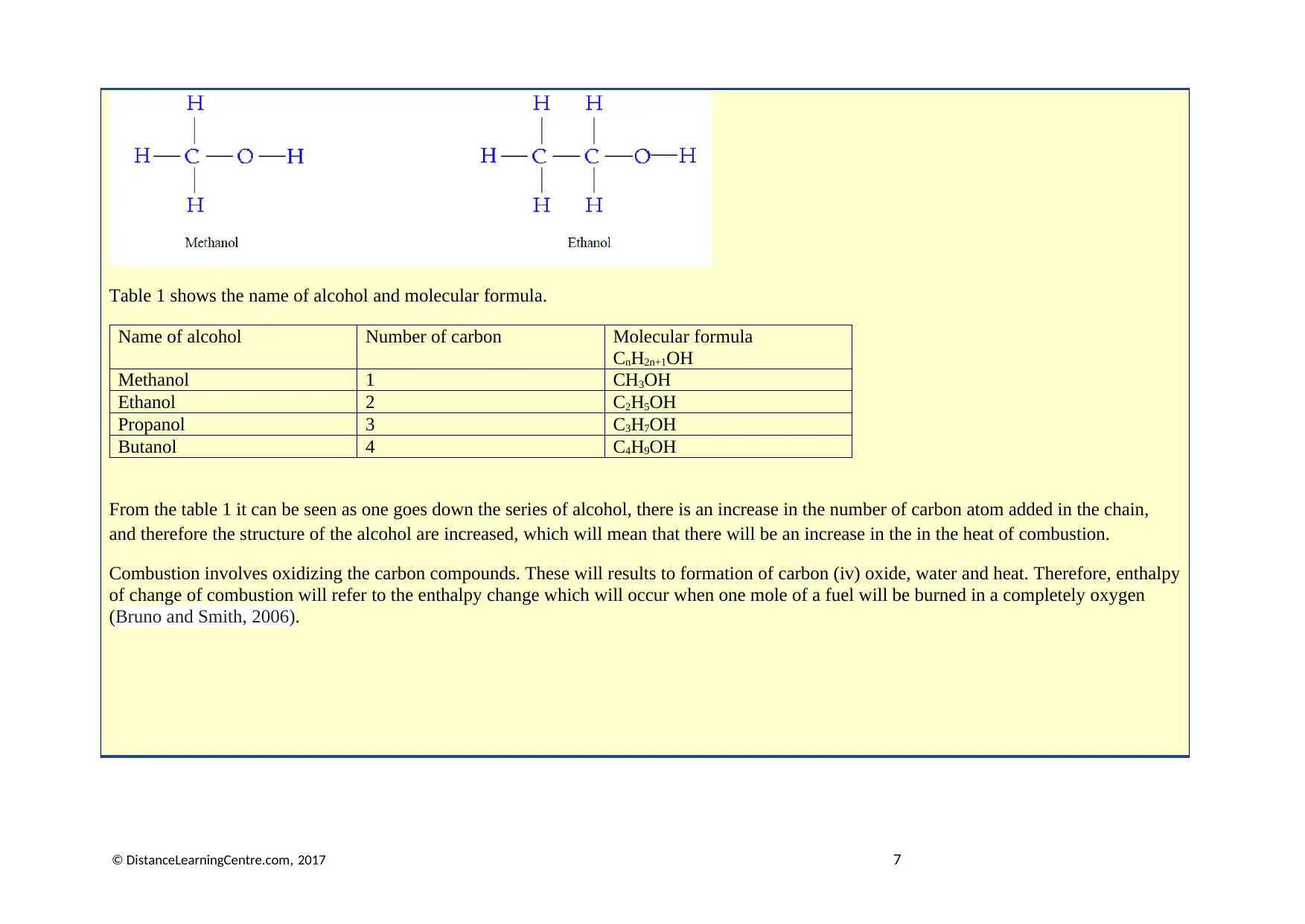
Table 1 shows the name of alcohol and molecular formula.
Name of alcohol Number of carbon Molecular formula
CnH2n+1OH
Methanol 1 CH3OH
Ethanol 2 C2H5OH
Propanol 3 C3H7OH
Butanol 4 C4H9OH
From the table 1 it can be seen as one goes down the series of alcohol, there is an increase in the number of carbon atom added in the chain,
and therefore the structure of the alcohol are increased, which will mean that there will be an increase in the in the heat of combustion.
Combustion involves oxidizing the carbon compounds. These will results to formation of carbon (iv) oxide, water and heat. Therefore, enthalpy
of change of combustion will refer to the enthalpy change which will occur when one mole of a fuel will be burned in a completely oxygen
(Bruno and Smith, 2006).
© DistanceLearningCentre.com, 2017 7
Name of alcohol Number of carbon Molecular formula
CnH2n+1OH
Methanol 1 CH3OH
Ethanol 2 C2H5OH
Propanol 3 C3H7OH
Butanol 4 C4H9OH
From the table 1 it can be seen as one goes down the series of alcohol, there is an increase in the number of carbon atom added in the chain,
and therefore the structure of the alcohol are increased, which will mean that there will be an increase in the in the heat of combustion.
Combustion involves oxidizing the carbon compounds. These will results to formation of carbon (iv) oxide, water and heat. Therefore, enthalpy
of change of combustion will refer to the enthalpy change which will occur when one mole of a fuel will be burned in a completely oxygen
(Bruno and Smith, 2006).
© DistanceLearningCentre.com, 2017 7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
"PROPOSED PLAN FOR THE INVESTIGATION"
AC "What you
need to do"
"Word
count
(approx)
"
"Table for you to complete"
2.4 "Review the
literature
which
already exists
about the
experimental
procedure.
Explain how
it will help
inform your
plan.
(You should
include in-
text
references
here)"
N/A In an enthalpy change experiment, the tested substance may undergo a chemical or a physical change,
during the process the system may either release or absorb the energy. The reaction that will involve a
system absorbing energy will be categorized as endothermic. The reaction that will involve in releasing the
heat will be classified as exothermic. Standard enthalpy is always determined under conditions that are
control; these conditions include both constant pressures of 1 atmosphere and at a room temperature at 25
degree Celsius. The enthalpy of any reaction in the laboratory will be determined using calorimeter. The
heat of reaction is determined using the application of Hess’s law, using the formula ∆ H0reaccrion =
∑ n ∆ Hfp−∑ n ∆ Hfr (Ozsezen and Canakci, 2011).
2.1 "List the
equipment
and
chemicals/rea
gents you
would use to
undertake
your
experiment."
50 List of equipment
Apparatus
1. 1 100 cm3 measuring Cylinder
2. 1 Electronic mass balance
3. 1 calorimeter
4. 1 thermometer
5. 4 Sprit lamps
© DistanceLearningCentre.com, 2017 8
AC "What you
need to do"
"Word
count
(approx)
"
"Table for you to complete"
2.4 "Review the
literature
which
already exists
about the
experimental
procedure.
Explain how
it will help
inform your
plan.
(You should
include in-
text
references
here)"
N/A In an enthalpy change experiment, the tested substance may undergo a chemical or a physical change,
during the process the system may either release or absorb the energy. The reaction that will involve a
system absorbing energy will be categorized as endothermic. The reaction that will involve in releasing the
heat will be classified as exothermic. Standard enthalpy is always determined under conditions that are
control; these conditions include both constant pressures of 1 atmosphere and at a room temperature at 25
degree Celsius. The enthalpy of any reaction in the laboratory will be determined using calorimeter. The
heat of reaction is determined using the application of Hess’s law, using the formula ∆ H0reaccrion =
∑ n ∆ Hfp−∑ n ∆ Hfr (Ozsezen and Canakci, 2011).
2.1 "List the
equipment
and
chemicals/rea
gents you
would use to
undertake
your
experiment."
50 List of equipment
Apparatus
1. 1 100 cm3 measuring Cylinder
2. 1 Electronic mass balance
3. 1 calorimeter
4. 1 thermometer
5. 4 Sprit lamps
© DistanceLearningCentre.com, 2017 8
6. Tripod stand
7. Stop watch
Chemical list
1. 100 cm3 distilled water
2. 200 cm3 of methanol
3. 200 cm3 of ethanol
4. 200 cm3 of propanol
5. 200 cm3 of butanol
2.1 "Complete
the Risk
assessment
below."
N/A All the alcohols under test are flammable, great care should be taken to avoid being burnt.
"RISK ASSESSMENT (No word count)"
"Column 1" "Column 2" "Column 3" "Column 4"
"Name of the chemical or hazard which may
be incurred."
"Risk
incurred"
"How will you reduce the severity of the risk?" "Risk reduced to"
All the alcohol
under test
Caution should be while carry out the experiment
throught
Prevention from
being burnt
"Record procedures to be employed to deal with:"
a) "Spillage in the lab
using larger measuring cylinders to ensure that no overflow takes place
immediate wiping of the area that has been wetted by the spillage
Using"
© DistanceLearningCentre.com, 2017 9
7. Stop watch
Chemical list
1. 100 cm3 distilled water
2. 200 cm3 of methanol
3. 200 cm3 of ethanol
4. 200 cm3 of propanol
5. 200 cm3 of butanol
2.1 "Complete
the Risk
assessment
below."
N/A All the alcohols under test are flammable, great care should be taken to avoid being burnt.
"RISK ASSESSMENT (No word count)"
"Column 1" "Column 2" "Column 3" "Column 4"
"Name of the chemical or hazard which may
be incurred."
"Risk
incurred"
"How will you reduce the severity of the risk?" "Risk reduced to"
All the alcohol
under test
Caution should be while carry out the experiment
throught
Prevention from
being burnt
"Record procedures to be employed to deal with:"
a) "Spillage in the lab
using larger measuring cylinders to ensure that no overflow takes place
immediate wiping of the area that has been wetted by the spillage
Using"
© DistanceLearningCentre.com, 2017 9
b) "Correct disposal of waste chemicals"
The chemical waste should be disposed well in the dustbin
"METHODOLOGY"
AC "What you
need to do"
"Word
count
(approx)
"
"Table for you to complete"
2.1/
2.3
"Provide a
detailed
method on
how you would
carry out the
experiment.
Make sure that
you identify
the variables
that need to be
controlled and
how this will
be done."
300 Procedures
1. Using electronic balances weigh the empty sprit lamp, and record the reading.
2. To the empty sprit lamp add about 40 cm3 of alcohol that is to be tested.
3. Using the electronic balance weigh the mass of the sprit lamp with the alcohol, record the reading.
4. Measure 100 cm3 of distilled water using the measuring cylinder
5. To the calorimeter add the measured water into a copper beaker.
6. Using the thermometer record the initial temperature of water
7. Insulate the calorimeter to prevent heat loss
8. After placing the thermometer and the stirrer inside the beaker cover the calorimeter using a lid
9. Light the sprit lump using a matchstick, when the alcohol starts burning start the stopwatch.
10. Stir the water throughout during the process
11. Record the temperature rise of water
12. Blow off the sprit lamp when the temperature rises to 330C
13. Stop the stopwatch instantly
14. Weigh the final mass of the sprit lamp containing the alcohol, record the readings.
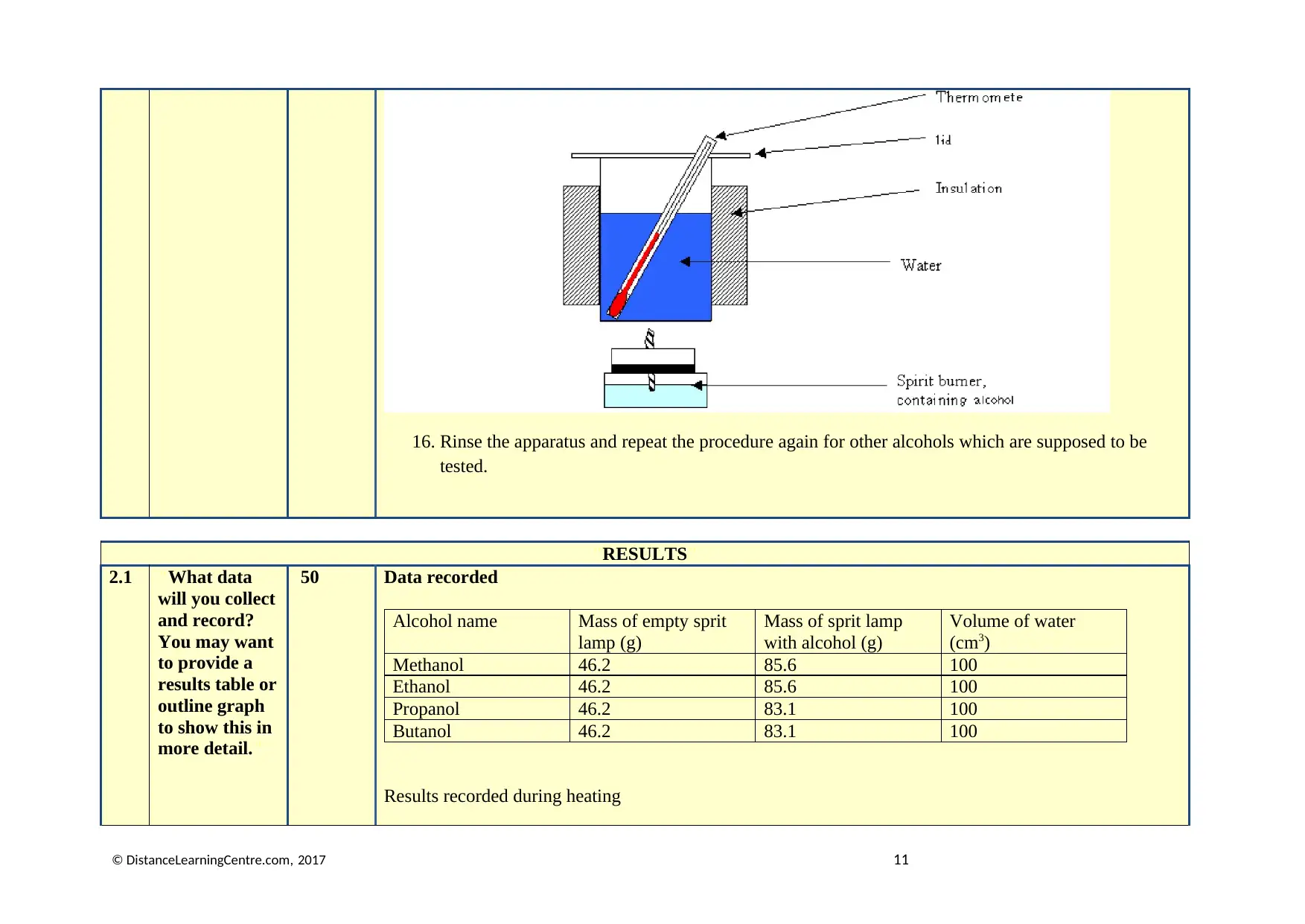
15. The setup should be similar to the following figure.
© DistanceLearningCentre.com, 2017 10
The chemical waste should be disposed well in the dustbin
"METHODOLOGY"
AC "What you
need to do"
"Word
count
(approx)
"
"Table for you to complete"
2.1/
2.3
"Provide a
detailed
method on
how you would
carry out the
experiment.
Make sure that
you identify
the variables
that need to be
controlled and
how this will
be done."
300 Procedures
1. Using electronic balances weigh the empty sprit lamp, and record the reading.
2. To the empty sprit lamp add about 40 cm3 of alcohol that is to be tested.
3. Using the electronic balance weigh the mass of the sprit lamp with the alcohol, record the reading.
4. Measure 100 cm3 of distilled water using the measuring cylinder
5. To the calorimeter add the measured water into a copper beaker.
6. Using the thermometer record the initial temperature of water
7. Insulate the calorimeter to prevent heat loss
8. After placing the thermometer and the stirrer inside the beaker cover the calorimeter using a lid
9. Light the sprit lump using a matchstick, when the alcohol starts burning start the stopwatch.
10. Stir the water throughout during the process
11. Record the temperature rise of water
12. Blow off the sprit lamp when the temperature rises to 330C
13. Stop the stopwatch instantly
14. Weigh the final mass of the sprit lamp containing the alcohol, record the readings.
15. The setup should be similar to the following figure.
© DistanceLearningCentre.com, 2017 10
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
16. Rinse the apparatus and repeat the procedure again for other alcohols which are supposed to be
tested.
"RESULTS"
2.1 "What data
will you collect
and record?
You may want
to provide a
results table or
outline graph
to show this in
more detail."
50 Data recorded
Alcohol name Mass of empty sprit
lamp (g)
Mass of sprit lamp
with alcohol (g)
Volume of water
(cm3)
Methanol 46.2 85.6 100
Ethanol 46.2 85.6 100
Propanol 46.2 83.1 100
Butanol 46.2 83.1 100
Results recorded during heating
© DistanceLearningCentre.com, 2017 11
tested.
"RESULTS"
2.1 "What data
will you collect
and record?
You may want
to provide a
results table or
outline graph
to show this in
more detail."
50 Data recorded
Alcohol name Mass of empty sprit
lamp (g)
Mass of sprit lamp
with alcohol (g)
Volume of water
(cm3)
Methanol 46.2 85.6 100
Ethanol 46.2 85.6 100
Propanol 46.2 83.1 100
Butanol 46.2 83.1 100
Results recorded during heating
© DistanceLearningCentre.com, 2017 11
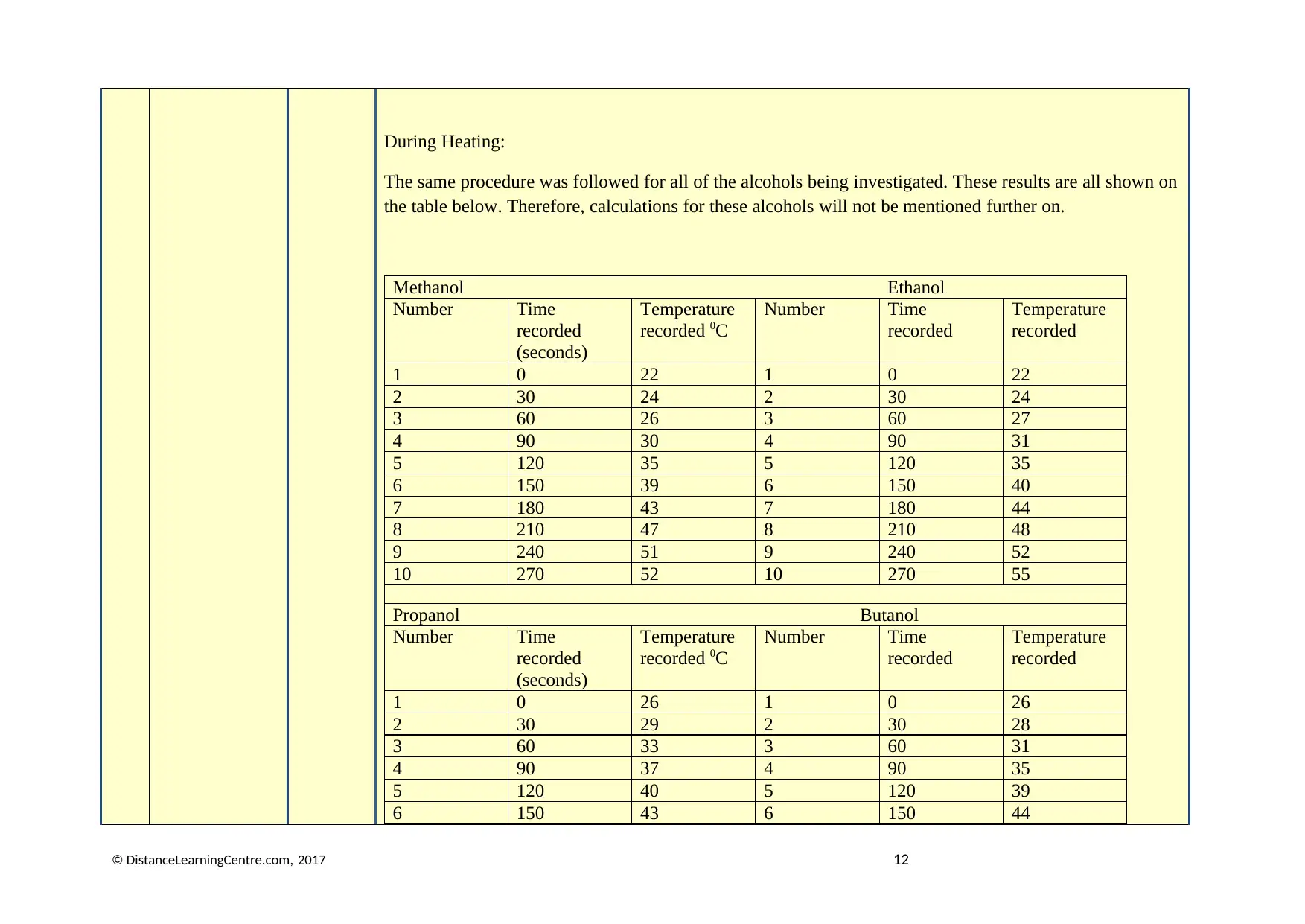
During Heating:
The same procedure was followed for all of the alcohols being investigated. These results are all shown on
the table below. Therefore, calculations for these alcohols will not be mentioned further on.
Methanol Ethanol
Number Time
recorded
(seconds)
Temperature
recorded 0C
Number Time
recorded
Temperature
recorded
1 0 22 1 0 22
2 30 24 2 30 24
3 60 26 3 60 27
4 90 30 4 90 31
5 120 35 5 120 35
6 150 39 6 150 40
7 180 43 7 180 44
8 210 47 8 210 48
9 240 51 9 240 52
10 270 52 10 270 55
Propanol Butanol
Number Time
recorded
(seconds)
Temperature
recorded 0C
Number Time
recorded
Temperature
recorded
1 0 26 1 0 26
2 30 29 2 30 28
3 60 33 3 60 31
4 90 37 4 90 35
5 120 40 5 120 39
6 150 43 6 150 44
© DistanceLearningCentre.com, 2017 12
The same procedure was followed for all of the alcohols being investigated. These results are all shown on
the table below. Therefore, calculations for these alcohols will not be mentioned further on.
Methanol Ethanol
Number Time
recorded
(seconds)
Temperature
recorded 0C
Number Time
recorded
Temperature
recorded
1 0 22 1 0 22
2 30 24 2 30 24
3 60 26 3 60 27
4 90 30 4 90 31
5 120 35 5 120 35
6 150 39 6 150 40
7 180 43 7 180 44
8 210 47 8 210 48
9 240 51 9 240 52
10 270 52 10 270 55
Propanol Butanol
Number Time
recorded
(seconds)
Temperature
recorded 0C
Number Time
recorded
Temperature
recorded
1 0 26 1 0 26
2 30 29 2 30 28
3 60 33 3 60 31
4 90 37 4 90 35
5 120 40 5 120 39
6 150 43 6 150 44
© DistanceLearningCentre.com, 2017 12
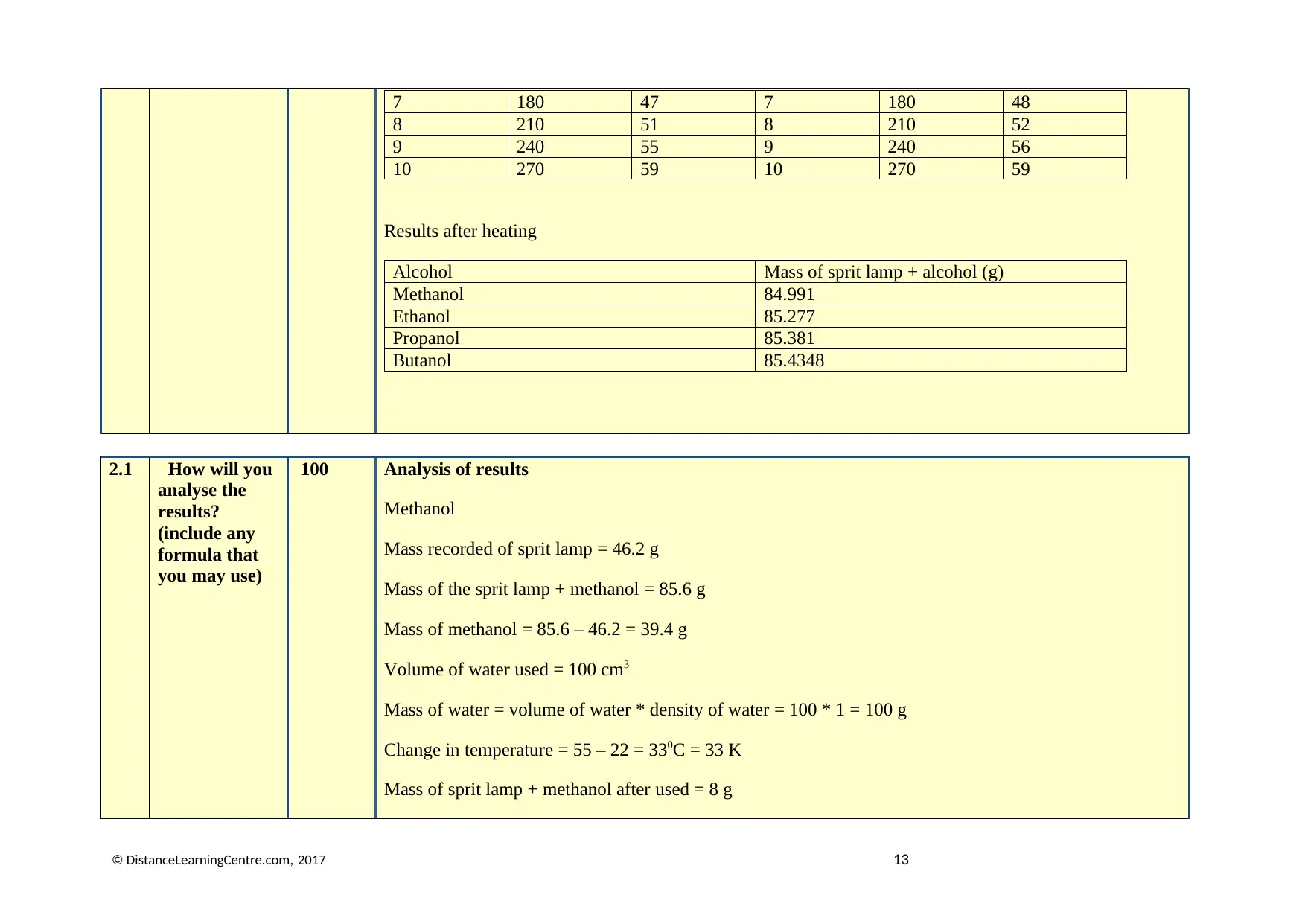
7 180 47 7 180 48
8 210 51 8 210 52
9 240 55 9 240 56
10 270 59 10 270 59
Results after heating
Alcohol Mass of sprit lamp + alcohol (g)
Methanol 84.991
Ethanol 85.277
Propanol 85.381
Butanol 85.4348
2.1 "How will you
analyse the
results?
(include any
formula that
you may use)"
100 Analysis of results
Methanol
Mass recorded of sprit lamp = 46.2 g
Mass of the sprit lamp + methanol = 85.6 g
Mass of methanol = 85.6 – 46.2 = 39.4 g
Volume of water used = 100 cm3
Mass of water = volume of water * density of water = 100 * 1 = 100 g
Change in temperature = 55 – 22 = 330C = 33 K
Mass of sprit lamp + methanol after used = 8 g
© DistanceLearningCentre.com, 2017 13
8 210 51 8 210 52
9 240 55 9 240 56
10 270 59 10 270 59
Results after heating
Alcohol Mass of sprit lamp + alcohol (g)
Methanol 84.991
Ethanol 85.277
Propanol 85.381
Butanol 85.4348
2.1 "How will you
analyse the
results?
(include any
formula that
you may use)"
100 Analysis of results
Methanol
Mass recorded of sprit lamp = 46.2 g
Mass of the sprit lamp + methanol = 85.6 g
Mass of methanol = 85.6 – 46.2 = 39.4 g
Volume of water used = 100 cm3
Mass of water = volume of water * density of water = 100 * 1 = 100 g
Change in temperature = 55 – 22 = 330C = 33 K
Mass of sprit lamp + methanol after used = 8 g
© DistanceLearningCentre.com, 2017 13
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
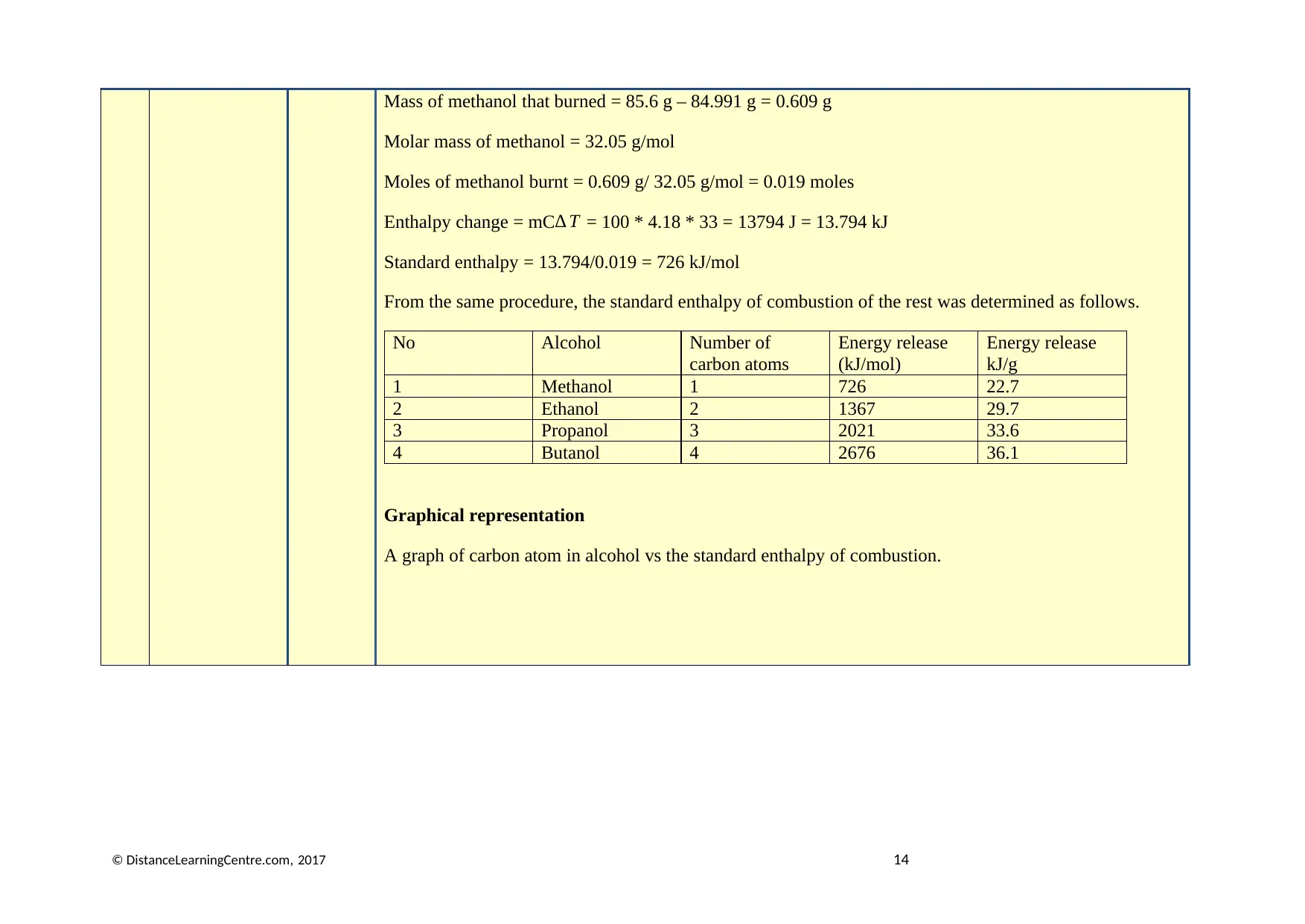
Mass of methanol that burned = 85.6 g – 84.991 g = 0.609 g
Molar mass of methanol = 32.05 g/mol
Moles of methanol burnt = 0.609 g/ 32.05 g/mol = 0.019 moles
Enthalpy change = mC ∆ T = 100 * 4.18 * 33 = 13794 J = 13.794 kJ
Standard enthalpy = 13.794/0.019 = 726 kJ/mol
From the same procedure, the standard enthalpy of combustion of the rest was determined as follows.
No Alcohol Number of
carbon atoms
Energy release
(kJ/mol)
Energy release
kJ/g
1 Methanol 1 726 22.7
2 Ethanol 2 1367 29.7
3 Propanol 3 2021 33.6
4 Butanol 4 2676 36.1
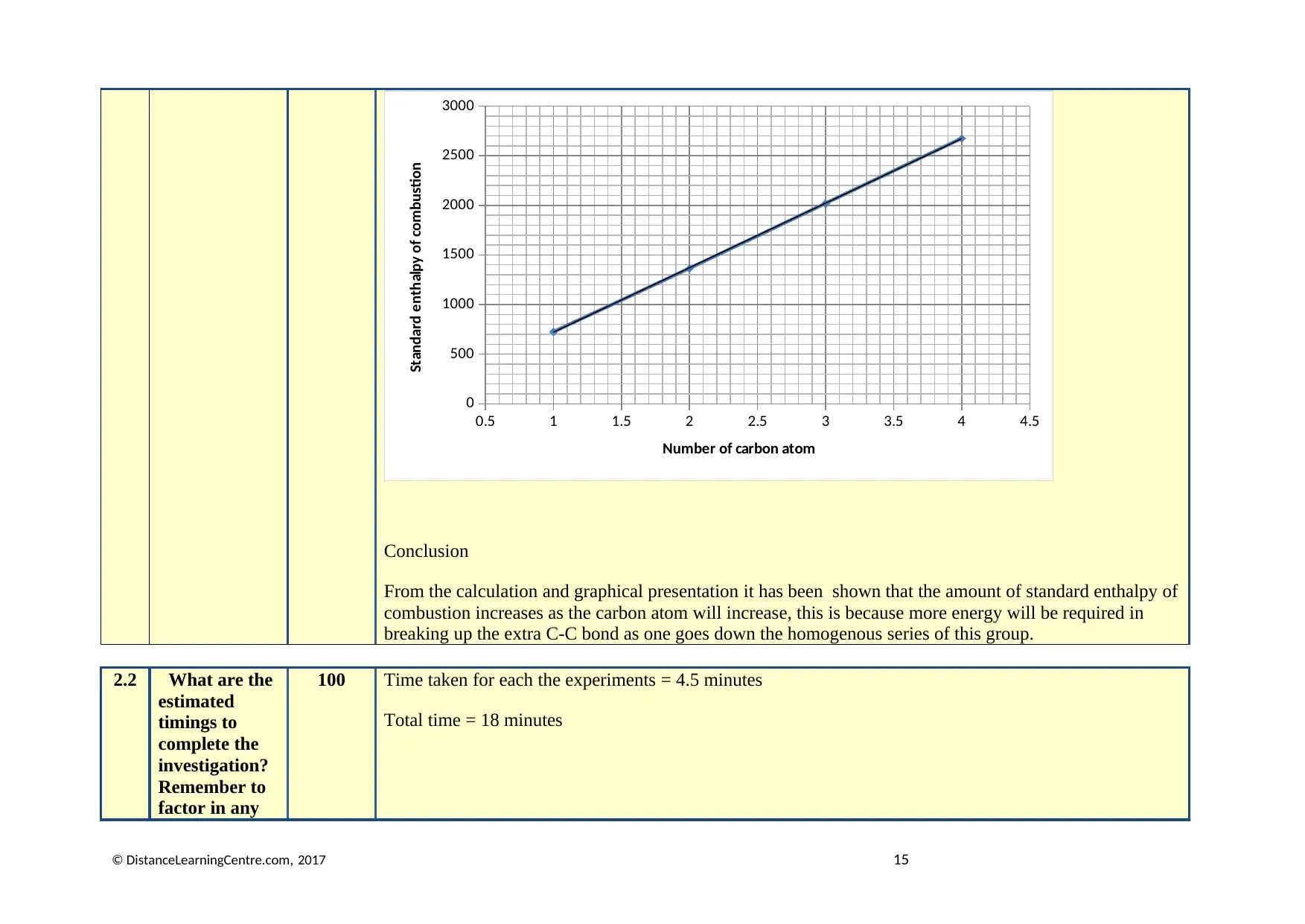
Graphical representation
A graph of carbon atom in alcohol vs the standard enthalpy of combustion.
© DistanceLearningCentre.com, 2017 14
Molar mass of methanol = 32.05 g/mol
Moles of methanol burnt = 0.609 g/ 32.05 g/mol = 0.019 moles
Enthalpy change = mC ∆ T = 100 * 4.18 * 33 = 13794 J = 13.794 kJ
Standard enthalpy = 13.794/0.019 = 726 kJ/mol
From the same procedure, the standard enthalpy of combustion of the rest was determined as follows.
No Alcohol Number of
carbon atoms
Energy release
(kJ/mol)
Energy release
kJ/g
1 Methanol 1 726 22.7
2 Ethanol 2 1367 29.7
3 Propanol 3 2021 33.6
4 Butanol 4 2676 36.1
Graphical representation
A graph of carbon atom in alcohol vs the standard enthalpy of combustion.
© DistanceLearningCentre.com, 2017 14
0.5 1 1.5 2 2.5 3 3.5 4 4.5
0
500
1000
1500
2000
2500
3000
Number of carbon atom
Standard enthalpy of combustion
Conclusion
From the calculation and graphical presentation it has been shown that the amount of standard enthalpy of
combustion increases as the carbon atom will increase, this is because more energy will be required in
breaking up the extra C-C bond as one goes down the homogenous series of this group.
2.2 "What are the
estimated
timings to
complete the
investigation?
Remember to
factor in any
100 Time taken for each the experiments = 4.5 minutes
Total time = 18 minutes
© DistanceLearningCentre.com, 2017 15
0
500
1000
1500
2000
2500
3000
Number of carbon atom
Standard enthalpy of combustion
Conclusion
From the calculation and graphical presentation it has been shown that the amount of standard enthalpy of
combustion increases as the carbon atom will increase, this is because more energy will be required in
breaking up the extra C-C bond as one goes down the homogenous series of this group.
2.2 "What are the
estimated
timings to
complete the
investigation?
Remember to
factor in any
100 Time taken for each the experiments = 4.5 minutes
Total time = 18 minutes
© DistanceLearningCentre.com, 2017 15
practical
considerations
eg leaving
overnight etc."
"REFERENCES / BIBLIOGRAPHY (2.4) (No word count)"
"
Bruno, T.J. and Smith, B.L., 2006. Enthalpy of combustion of fuels as a function of distillate cut: Application of an advanced distillation curve
method. Energy & fuels, 20(5), pp.2109-2116.
Ozsezen, A.N. and Canakci, M., 2011. Performance and combustion characteristics of alcohol–gasoline blends at wide-open
throttle. Energy, 36(5), pp.2747-2752.
Hellier, P., Ladommatos, N., Allan, R. and Rogerson, J., 2012. The influence of fatty acid ester alcohol moiety molecular structure on diesel
combustion and emissions. Energy & fuels, 26(3), pp.1912-1927.
© DistanceLearningCentre.com, 2017 16
considerations
eg leaving
overnight etc."
"REFERENCES / BIBLIOGRAPHY (2.4) (No word count)"
"
Bruno, T.J. and Smith, B.L., 2006. Enthalpy of combustion of fuels as a function of distillate cut: Application of an advanced distillation curve
method. Energy & fuels, 20(5), pp.2109-2116.
Ozsezen, A.N. and Canakci, M., 2011. Performance and combustion characteristics of alcohol–gasoline blends at wide-open
throttle. Energy, 36(5), pp.2747-2752.
Hellier, P., Ladommatos, N., Allan, R. and Rogerson, J., 2012. The influence of fatty acid ester alcohol moiety molecular structure on diesel
combustion and emissions. Energy & fuels, 26(3), pp.1912-1927.
© DistanceLearningCentre.com, 2017 16
1 out of 16
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.





