HER-2 Positive Breast Cancer
VerifiedAdded on 2023/04/21
|16
|4060
|499
AI Summary
HER-2 positive breast cancer is a type of breast cancer characterized by the overexpression of the HER-2 gene. This leads to uncontrolled cellular growth and proliferation, resulting in an aggressive form of cancer. This article discusses the causes, diagnosis, and treatment options for HER-2 positive breast cancer. Find study material, solved assignments, and essays on HER-2 positive breast cancer at Desklib.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: HER-2 POSITIVE BREAST CANCER
HER-2 Positive Breast Cancer
Name of student:
Name of university:
Author Note:
HER-2 Positive Breast Cancer
Name of student:
Name of university:
Author Note:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

1HER-2 POSITIVE BREAST CANCER
Introduction
HER-2 positive breast cancers are characterized by a mutation in Human epidermal
growth factor Receptor 2 (HER-2) which shows a manifestation of tumor growth through
excessive cellular growth and proliferation. Mutations in HER-2 gene result in aggressive
form of breast cancers. Recurrence of breast cancers can be a sign of HER-2 overexpression
(Kümler, Tuxen and Nielsen 2014). The precise causative factors of breast carcinomas are
debatable and yet doubtful. Still, breast cancer risks in women increases through increased
exposure to hormones like estrogen and progesterone. More estrogen in cellular system
increases the susceptibility of women to breast cancers.
HER-2 positive breast cancer
Human Epidermal growth factor Receptor (HER) is a family of receptors which
consist of four members such as HER-1, hER-2, HER-3 and HER-4 which are also
recognized as ErbB1, ErbB2, ErbB3 and ErbB4 respectively. The pathogenicity of breast
cancer shows the involvement of HER-2in inducing carcinogenicity of mammary glands. The
amplification of HER-2 gene leading to overexpression has been found in 15-30 % of
reported breast cancers (Kümler, Tuxen and Nielsen 2014). HER-2 has been found to be
present in multiple copies in breast cancers; the expression of HER-2 gene leads to increased
protein expression which in turn leads to multiple receptors to be expressed on the tumor cell
surface. The estrogen binds to its estrogen receptor and brings about activation of HER-2
signaling cascade. The progression of breast cancer from stage I to stage III has been
correlated with increased overexpression of HER-2 in case of invasive metastasis (Kümler,
Tuxen and Nielsen 2014). P95 is an abnormal form of Her-2 which has been found to be
constitutively active in breast cancers; this aberrant p95 form of HER-2 lacks the
extracellular domain. Her-positive breast cancers have an aggressive form of carcinogenicity
Introduction
HER-2 positive breast cancers are characterized by a mutation in Human epidermal
growth factor Receptor 2 (HER-2) which shows a manifestation of tumor growth through
excessive cellular growth and proliferation. Mutations in HER-2 gene result in aggressive
form of breast cancers. Recurrence of breast cancers can be a sign of HER-2 overexpression
(Kümler, Tuxen and Nielsen 2014). The precise causative factors of breast carcinomas are
debatable and yet doubtful. Still, breast cancer risks in women increases through increased
exposure to hormones like estrogen and progesterone. More estrogen in cellular system
increases the susceptibility of women to breast cancers.
HER-2 positive breast cancer
Human Epidermal growth factor Receptor (HER) is a family of receptors which
consist of four members such as HER-1, hER-2, HER-3 and HER-4 which are also
recognized as ErbB1, ErbB2, ErbB3 and ErbB4 respectively. The pathogenicity of breast
cancer shows the involvement of HER-2in inducing carcinogenicity of mammary glands. The
amplification of HER-2 gene leading to overexpression has been found in 15-30 % of
reported breast cancers (Kümler, Tuxen and Nielsen 2014). HER-2 has been found to be
present in multiple copies in breast cancers; the expression of HER-2 gene leads to increased
protein expression which in turn leads to multiple receptors to be expressed on the tumor cell
surface. The estrogen binds to its estrogen receptor and brings about activation of HER-2
signaling cascade. The progression of breast cancer from stage I to stage III has been
correlated with increased overexpression of HER-2 in case of invasive metastasis (Kümler,
Tuxen and Nielsen 2014). P95 is an abnormal form of Her-2 which has been found to be
constitutively active in breast cancers; this aberrant p95 form of HER-2 lacks the
extracellular domain. Her-positive breast cancers have an aggressive form of carcinogenicity

2HER-2 POSITIVE BREAST CANCER
compared to HER-2 negative breast cancers. Her-2 overexpression leads to uncontrolled
cellular growth and proliferation, finally leading to cancerous development.
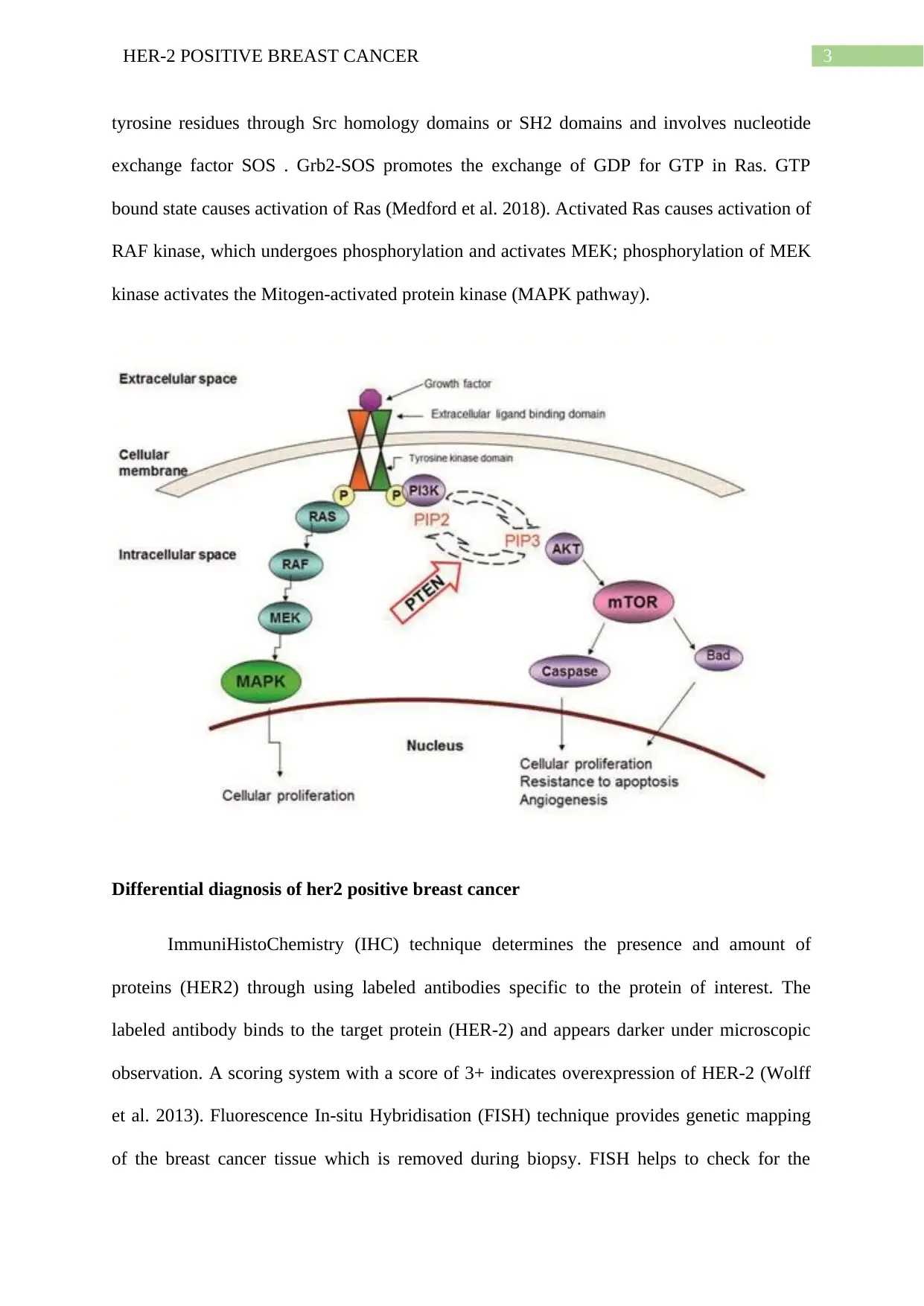
Signaling
HER family of receptors lead to downstream signaling cascade through receptor
homodimerisation or heterodimerisation, ultimately leading to increased cellular growth and
proliferation. HER-2 with its kinase domain remains present in an open conformation; HER-2
dimerises with either HER1 or HER-3 and activates the PI3K and AKT downstream
signaling which further activates Raf and MAPK signaling pathways. HER2 itself cannot
activate PI3K/Akt signaling pathway in tumor progression; dimerisation of HER-2 with
HER-3 causes an open conformational state exposing the extracellular dimerisation domain
(Paplomata and O’Regan 2014). This indirect mechanism of HER2-HER-3 dimerisation
takes place through second messenger pathways like Grb2 and Ras signaling activation.
HER2-HER-3 dimerisation causes phosphorylation of tyrosine kinase domains in plasma
membrane; the Grb2 adaptor molecule bind sto phosphorylated tyrosine kinase and activates
Ras protein through exchange of GDP to GTP state. This activated PI3K causes transfer of
phosphate groups from PIP2 to PIP3 and ultimately leading to Phosphatidyl inositol 3,4,5
triphosphate formation. PIP3, anchored to the plasma membrane, binds to the plextrin
homology (PH) domain of Akt, causing its activation (Yang, Polley and Lipkowitz 2016.).
This allows translocation of Akt from plasma membrane to cytosol, followed by
conformational changes and phosphorylation of threonine 308 by PDK1 and serine 473 by
PDK2. Akt then enters into nucleus and performs transcription regulation of proteins through
phosphorylation.
HER-2 receptor dimerisation causes phosphorylation of tyrosine residues on the
cytoplasmic kinase domain. Docking proteins like grb2 binds to these phosphorylated
compared to HER-2 negative breast cancers. Her-2 overexpression leads to uncontrolled
cellular growth and proliferation, finally leading to cancerous development.
Signaling
HER family of receptors lead to downstream signaling cascade through receptor
homodimerisation or heterodimerisation, ultimately leading to increased cellular growth and
proliferation. HER-2 with its kinase domain remains present in an open conformation; HER-2
dimerises with either HER1 or HER-3 and activates the PI3K and AKT downstream
signaling which further activates Raf and MAPK signaling pathways. HER2 itself cannot
activate PI3K/Akt signaling pathway in tumor progression; dimerisation of HER-2 with
HER-3 causes an open conformational state exposing the extracellular dimerisation domain
(Paplomata and O’Regan 2014). This indirect mechanism of HER2-HER-3 dimerisation
takes place through second messenger pathways like Grb2 and Ras signaling activation.
HER2-HER-3 dimerisation causes phosphorylation of tyrosine kinase domains in plasma
membrane; the Grb2 adaptor molecule bind sto phosphorylated tyrosine kinase and activates
Ras protein through exchange of GDP to GTP state. This activated PI3K causes transfer of
phosphate groups from PIP2 to PIP3 and ultimately leading to Phosphatidyl inositol 3,4,5
triphosphate formation. PIP3, anchored to the plasma membrane, binds to the plextrin
homology (PH) domain of Akt, causing its activation (Yang, Polley and Lipkowitz 2016.).
This allows translocation of Akt from plasma membrane to cytosol, followed by
conformational changes and phosphorylation of threonine 308 by PDK1 and serine 473 by
PDK2. Akt then enters into nucleus and performs transcription regulation of proteins through
phosphorylation.
HER-2 receptor dimerisation causes phosphorylation of tyrosine residues on the
cytoplasmic kinase domain. Docking proteins like grb2 binds to these phosphorylated
3HER-2 POSITIVE BREAST CANCER
tyrosine residues through Src homology domains or SH2 domains and involves nucleotide
exchange factor SOS . Grb2-SOS promotes the exchange of GDP for GTP in Ras. GTP
bound state causes activation of Ras (Medford et al. 2018). Activated Ras causes activation of
RAF kinase, which undergoes phosphorylation and activates MEK; phosphorylation of MEK
kinase activates the Mitogen-activated protein kinase (MAPK pathway).
Differential diagnosis of her2 positive breast cancer
ImmuniHistoChemistry (IHC) technique determines the presence and amount of
proteins (HER2) through using labeled antibodies specific to the protein of interest. The
labeled antibody binds to the target protein (HER-2) and appears darker under microscopic
observation. A scoring system with a score of 3+ indicates overexpression of HER-2 (Wolff
et al. 2013). Fluorescence In-situ Hybridisation (FISH) technique provides genetic mapping
of the breast cancer tissue which is removed during biopsy. FISH helps to check for the
tyrosine residues through Src homology domains or SH2 domains and involves nucleotide
exchange factor SOS . Grb2-SOS promotes the exchange of GDP for GTP in Ras. GTP
bound state causes activation of Ras (Medford et al. 2018). Activated Ras causes activation of
RAF kinase, which undergoes phosphorylation and activates MEK; phosphorylation of MEK
kinase activates the Mitogen-activated protein kinase (MAPK pathway).
Differential diagnosis of her2 positive breast cancer
ImmuniHistoChemistry (IHC) technique determines the presence and amount of
proteins (HER2) through using labeled antibodies specific to the protein of interest. The
labeled antibody binds to the target protein (HER-2) and appears darker under microscopic
observation. A scoring system with a score of 3+ indicates overexpression of HER-2 (Wolff
et al. 2013). Fluorescence In-situ Hybridisation (FISH) technique provides genetic mapping
of the breast cancer tissue which is removed during biopsy. FISH helps to check for the
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

4HER-2 POSITIVE BREAST CANCER
presence of multiple copies of HER2 gene. Special colored dyes are attached to chromosomes
in patient tissue samples and visualized under fluorescent microscope (Wolff et al. 2013).
Chromogenic In-situ Hybridisation (CISH) technique uses a stain which shows a color
change of HER-2 genes in the tumor tissue sample. The cancer dells which have multiple
copies of HER-2 show color changes which are observed under the microscope.
Molecular subtypes of breast cancers
Luminal A breast cancer is defined as either estrogen or progesterone positive subtype
but HDER-2 negative containing high levels of Ki-67 protein; this has a slow progression.
Luminal B breast cancers are either estrogen or progesterone positive and also HER-2
positive or negative expressing high Ki-67 protein levels, having faster progression (Prat et.
2015). Triple negative (ER, PR and HER-2 negative) is predominant in women with BRCA1
mutations leading to carcinogenicity (Prat et. 2015). HER-2 overexpressed breast cancer
shows a faster progression due to uncontrolled cellular growth and proliferation.
presence of multiple copies of HER2 gene. Special colored dyes are attached to chromosomes
in patient tissue samples and visualized under fluorescent microscope (Wolff et al. 2013).
Chromogenic In-situ Hybridisation (CISH) technique uses a stain which shows a color
change of HER-2 genes in the tumor tissue sample. The cancer dells which have multiple
copies of HER-2 show color changes which are observed under the microscope.
Molecular subtypes of breast cancers
Luminal A breast cancer is defined as either estrogen or progesterone positive subtype
but HDER-2 negative containing high levels of Ki-67 protein; this has a slow progression.
Luminal B breast cancers are either estrogen or progesterone positive and also HER-2
positive or negative expressing high Ki-67 protein levels, having faster progression (Prat et.
2015). Triple negative (ER, PR and HER-2 negative) is predominant in women with BRCA1
mutations leading to carcinogenicity (Prat et. 2015). HER-2 overexpressed breast cancer
shows a faster progression due to uncontrolled cellular growth and proliferation.

5HER-2 POSITIVE BREAST CANCER
References
Kümler, I., Tuxen, M.K. and Nielsen, D.L., 2014. A systematic review of dual targeting in
HER2-positive breast cancer. Cancer treatment reviews, 40(2), pp.259-270.
Medford, A., Niemierko, A., Moy, B., Spring, L., Malvarosa, G., Younger, J., Lanman, R.B.,
Nagy, R.J., Corcoran, R.B., Isakoff, S.J. and Ellisen, L.W., 2018. Molecular alterations in the
Ras-Raf-Erk (MAPK) pathway in metastatic hormone receptor positive (HR+)/HER2-breast
cancer: Incidence and impact on clinical outcomes.
Paplomata, E. and O’Regan, R., 2014. The PI3K/AKT/mTOR pathway in breast cancer:
targets, trials and biomarkers. Therapeutic advances in medical oncology, 6(4), pp.154-166.
Prat, A., Pineda, E., Adamo, B., Galván, P., Fernández, A., Gaba, L., Díez, M., Viladot, M.,
Arance, A. and Muñoz, M., 2015. Clinical implications of the intrinsic molecular subtypes of
breast cancer. The Breast, 24, pp.S26-S35.
Wolff, A.C., Hammond, M.E.H., Hicks, D.G., Dowsett, M., McShane, L.M., Allison, K.H.,
Allred, D.C., Bartlett, J.M., Bilous, M., Fitzgibbons, P. and Hanna, W., 2013.
Recommendations for human epidermal growth factor receptor 2 testing in breast cancer:
American Society of Clinical Oncology/College of American Pathologists clinical practice
guideline update. Archives of Pathology and Laboratory Medicine, 138(2), pp.241-256.
Yang, S.X., Polley, E. and Lipkowitz, S., 2016. New insights on PI3K/AKT pathway
alterations and clinical outcomes in breast cancer. Cancer treatment reviews, 45, pp.87-96.
References
Kümler, I., Tuxen, M.K. and Nielsen, D.L., 2014. A systematic review of dual targeting in
HER2-positive breast cancer. Cancer treatment reviews, 40(2), pp.259-270.
Medford, A., Niemierko, A., Moy, B., Spring, L., Malvarosa, G., Younger, J., Lanman, R.B.,
Nagy, R.J., Corcoran, R.B., Isakoff, S.J. and Ellisen, L.W., 2018. Molecular alterations in the
Ras-Raf-Erk (MAPK) pathway in metastatic hormone receptor positive (HR+)/HER2-breast
cancer: Incidence and impact on clinical outcomes.
Paplomata, E. and O’Regan, R., 2014. The PI3K/AKT/mTOR pathway in breast cancer:
targets, trials and biomarkers. Therapeutic advances in medical oncology, 6(4), pp.154-166.
Prat, A., Pineda, E., Adamo, B., Galván, P., Fernández, A., Gaba, L., Díez, M., Viladot, M.,
Arance, A. and Muñoz, M., 2015. Clinical implications of the intrinsic molecular subtypes of
breast cancer. The Breast, 24, pp.S26-S35.
Wolff, A.C., Hammond, M.E.H., Hicks, D.G., Dowsett, M., McShane, L.M., Allison, K.H.,
Allred, D.C., Bartlett, J.M., Bilous, M., Fitzgibbons, P. and Hanna, W., 2013.
Recommendations for human epidermal growth factor receptor 2 testing in breast cancer:
American Society of Clinical Oncology/College of American Pathologists clinical practice
guideline update. Archives of Pathology and Laboratory Medicine, 138(2), pp.241-256.
Yang, S.X., Polley, E. and Lipkowitz, S., 2016. New insights on PI3K/AKT pathway
alterations and clinical outcomes in breast cancer. Cancer treatment reviews, 45, pp.87-96.

6HER-2 POSITIVE BREAST CANCER
Part B:
HER2 positive breast cancer is a very common breast cancer which affects more than
25% of women developing breast cancer. HER2 refers to a growth promoting factors that is
found in the cellular repair of the breast cells. The HER2 protein or factor is coded by the
HER2 gene and these receptor proteins help in controlling the growth and repair of the breast
cells. as discussed by Janiszewska et al. (2015), the over- expression of this gene leads to the
uncontrolled growth and cellular division of the breast cells and leads to the rather aggressive
form of breast cancer. The HER2 positive breast cancer refers to the over- expression of the
HER2 gene and it is a very aggressive and critical breast cancer for treatment and
management. Although, there are various different treatment modalities, that are now being
introduced to properly managing the HER2 positive breast cancers.
Aside from surgical intervention or radiation therapy, another very successful
treatment intervention that has been identified for the HER2 positive breast cancer is the
targeted combination drug therapy involving drugs like trastuzumab, and docetaxel. There is
mounting evidence which indicates at the effectiveness of these medications used in
conjunction to control the metastasis and target the over-expressing HER2 gene. Trastuzumab
refers to the most important and impact medication of this treatment regimen which helps in
blocking the metastatic cells from receiving the chemical signals entirely that triggers
uncontrollable growth. Although, there are considerable evidence which indicates at the
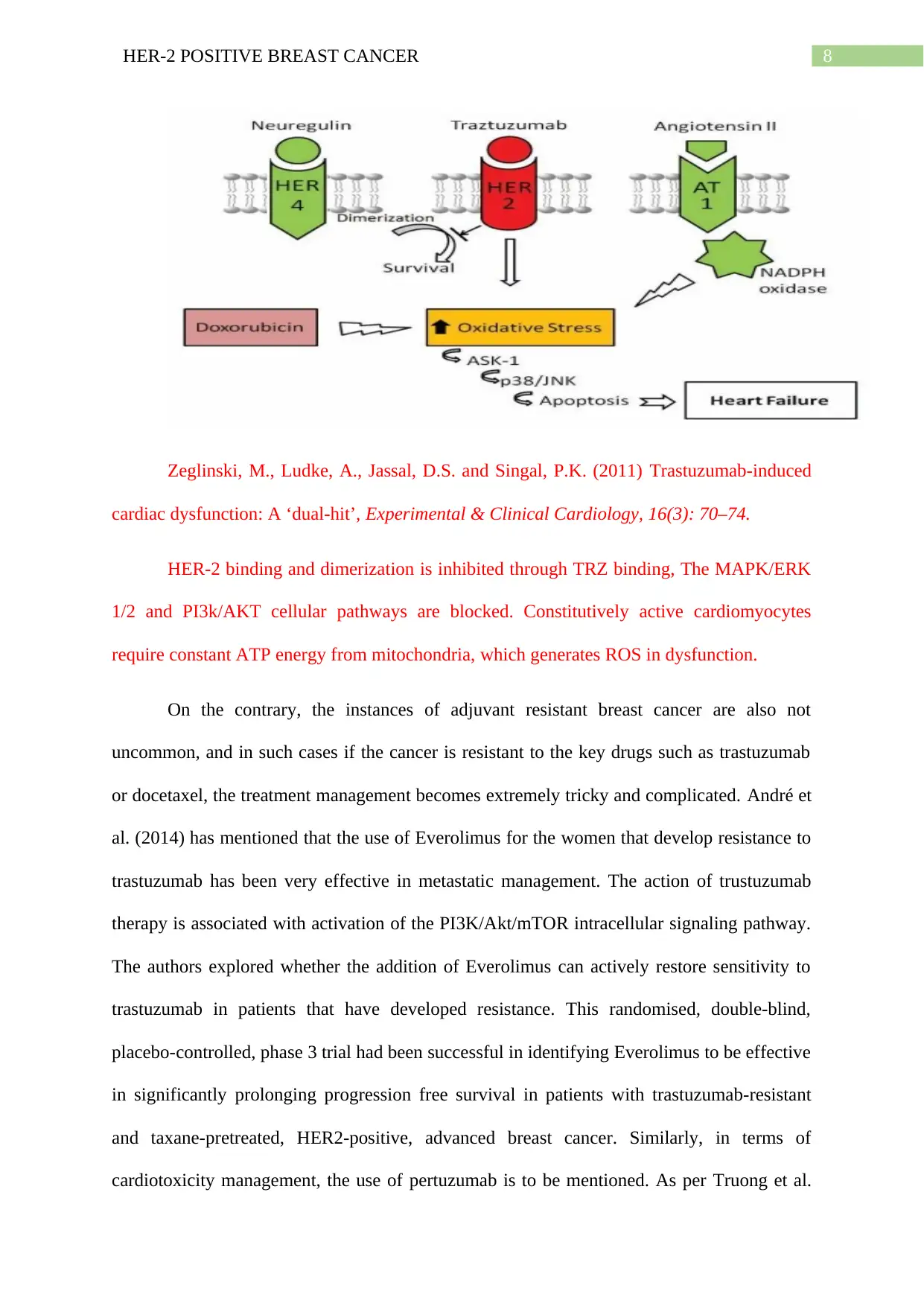
toxicity and negative effects of the disease to be overwhelming for the patients. Trz binding
to HER-2 is linked to downregulation of anti-apoptotic factors (BCL-XL) and upregulation of
BCL-XS. Therefore, a change in pro-apoptotic to anti-apoptotic ratio results in mitochondrial
dysfunction, finally leading to cardiomyocyte apoptosis. This blocking of MAPK/ERK1/2
and PI3K/AKT signalling cascades, accumulation of ROS in cardiomyocytes causes cardiac
dysfunction (Eschenhagen et al. 2011). A drug combination therapy with DOX is also
Part B:
HER2 positive breast cancer is a very common breast cancer which affects more than
25% of women developing breast cancer. HER2 refers to a growth promoting factors that is
found in the cellular repair of the breast cells. The HER2 protein or factor is coded by the
HER2 gene and these receptor proteins help in controlling the growth and repair of the breast
cells. as discussed by Janiszewska et al. (2015), the over- expression of this gene leads to the
uncontrolled growth and cellular division of the breast cells and leads to the rather aggressive
form of breast cancer. The HER2 positive breast cancer refers to the over- expression of the
HER2 gene and it is a very aggressive and critical breast cancer for treatment and
management. Although, there are various different treatment modalities, that are now being
introduced to properly managing the HER2 positive breast cancers.
Aside from surgical intervention or radiation therapy, another very successful
treatment intervention that has been identified for the HER2 positive breast cancer is the
targeted combination drug therapy involving drugs like trastuzumab, and docetaxel. There is
mounting evidence which indicates at the effectiveness of these medications used in
conjunction to control the metastasis and target the over-expressing HER2 gene. Trastuzumab
refers to the most important and impact medication of this treatment regimen which helps in
blocking the metastatic cells from receiving the chemical signals entirely that triggers
uncontrollable growth. Although, there are considerable evidence which indicates at the
toxicity and negative effects of the disease to be overwhelming for the patients. Trz binding
to HER-2 is linked to downregulation of anti-apoptotic factors (BCL-XL) and upregulation of
BCL-XS. Therefore, a change in pro-apoptotic to anti-apoptotic ratio results in mitochondrial
dysfunction, finally leading to cardiomyocyte apoptosis. This blocking of MAPK/ERK1/2
and PI3K/AKT signalling cascades, accumulation of ROS in cardiomyocytes causes cardiac
dysfunction (Eschenhagen et al. 2011). A drug combination therapy with DOX is also
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7HER-2 POSITIVE BREAST CANCER
resultant of ROS formation and oxidative stress. This causes up regulation of Angiotensin II
which inhibits NRG binding to HER receptors. This is in turn linked to ROS accumulation
within the heart, blocking cell survival pathways. Angiotensin II directs NADPH oxidase
activation through PKC dependent pathway. This causes superoxide generation in
mitochondria causing mitochondrial dysfunction and cellular death. This vicious cycle leads
to activation of AT1 signalling which activates p38 and jun N-linked kinase (Onitilo et al.
2014). Both these pathways are active during DOX treatment, participating in apoptotic
pathway and dysfunction. ***On the other hand, the alternative medication Kadcyla or
trastuzumab emtansine, has been proved to be more effective and efficient among the
patients, especially that have retained the disease even after initial drug treatment with just
trastuzumab (Tolaney et al. 2015). Moreover, researchers have also evidenced in the
KATHERINE clinical trial that the use of kadcyla in place of the standard trastuzumab had
been more effective to not just cure the cancer but also reduce the chances of reoccurrences in
the patients. Hence, it can be stated that the combination therapy using trastuzumab with the
chemotherapy agent emantisine helps in better control of the metastasis and can even
obliterate the risk of recurrence to a large extent. Although, argumentatively, the
CLEOPATRA trial by Sandra et al. (2015) has evidenced the use three drugs, Pertuzumab,
trastuzumab and Decotaxel to control and manage the HER2 metastatic cancer. As per the
article, the authors mentioned first-line therapy with pertuzumab, trastuzumab, and docetaxel
had been highly effective in improving the progression free survival rate among the target
population having HER2 positive breast cancer much more effectively and efficiently as
compared to the standard of only trastuzumab and docetaxel. The findings suggest the
addition of the pertuzumab to the standard combination drug therapy of trustuzumab and
docetaxel results on better and long term control of the disease.
resultant of ROS formation and oxidative stress. This causes up regulation of Angiotensin II
which inhibits NRG binding to HER receptors. This is in turn linked to ROS accumulation
within the heart, blocking cell survival pathways. Angiotensin II directs NADPH oxidase
activation through PKC dependent pathway. This causes superoxide generation in
mitochondria causing mitochondrial dysfunction and cellular death. This vicious cycle leads
to activation of AT1 signalling which activates p38 and jun N-linked kinase (Onitilo et al.
2014). Both these pathways are active during DOX treatment, participating in apoptotic
pathway and dysfunction. ***On the other hand, the alternative medication Kadcyla or
trastuzumab emtansine, has been proved to be more effective and efficient among the
patients, especially that have retained the disease even after initial drug treatment with just
trastuzumab (Tolaney et al. 2015). Moreover, researchers have also evidenced in the
KATHERINE clinical trial that the use of kadcyla in place of the standard trastuzumab had
been more effective to not just cure the cancer but also reduce the chances of reoccurrences in
the patients. Hence, it can be stated that the combination therapy using trastuzumab with the
chemotherapy agent emantisine helps in better control of the metastasis and can even
obliterate the risk of recurrence to a large extent. Although, argumentatively, the
CLEOPATRA trial by Sandra et al. (2015) has evidenced the use three drugs, Pertuzumab,
trastuzumab and Decotaxel to control and manage the HER2 metastatic cancer. As per the
article, the authors mentioned first-line therapy with pertuzumab, trastuzumab, and docetaxel
had been highly effective in improving the progression free survival rate among the target
population having HER2 positive breast cancer much more effectively and efficiently as
compared to the standard of only trastuzumab and docetaxel. The findings suggest the
addition of the pertuzumab to the standard combination drug therapy of trustuzumab and
docetaxel results on better and long term control of the disease.
8HER-2 POSITIVE BREAST CANCER
Zeglinski, M., Ludke, A., Jassal, D.S. and Singal, P.K. (2011) Trastuzumab-induced
cardiac dysfunction: A ‘dual-hit’, Experimental & Clinical Cardiology, 16(3): 70–74.
HER-2 binding and dimerization is inhibited through TRZ binding, The MAPK/ERK
1/2 and PI3k/AKT cellular pathways are blocked. Constitutively active cardiomyocytes
require constant ATP energy from mitochondria, which generates ROS in dysfunction.
On the contrary, the instances of adjuvant resistant breast cancer are also not
uncommon, and in such cases if the cancer is resistant to the key drugs such as trastuzumab
or docetaxel, the treatment management becomes extremely tricky and complicated. André et
al. (2014) has mentioned that the use of Everolimus for the women that develop resistance to
trastuzumab has been very effective in metastatic management. The action of trustuzumab
therapy is associated with activation of the PI3K/Akt/mTOR intracellular signaling pathway.
The authors explored whether the addition of Everolimus can actively restore sensitivity to
trastuzumab in patients that have developed resistance. This randomised, double-blind,
placebo-controlled, phase 3 trial had been successful in identifying Everolimus to be effective
in significantly prolonging progression free survival in patients with trastuzumab-resistant
and taxane-pretreated, HER2-positive, advanced breast cancer. Similarly, in terms of
cardiotoxicity management, the use of pertuzumab is to be mentioned. As per Truong et al.
Zeglinski, M., Ludke, A., Jassal, D.S. and Singal, P.K. (2011) Trastuzumab-induced
cardiac dysfunction: A ‘dual-hit’, Experimental & Clinical Cardiology, 16(3): 70–74.
HER-2 binding and dimerization is inhibited through TRZ binding, The MAPK/ERK
1/2 and PI3k/AKT cellular pathways are blocked. Constitutively active cardiomyocytes
require constant ATP energy from mitochondria, which generates ROS in dysfunction.
On the contrary, the instances of adjuvant resistant breast cancer are also not
uncommon, and in such cases if the cancer is resistant to the key drugs such as trastuzumab
or docetaxel, the treatment management becomes extremely tricky and complicated. André et
al. (2014) has mentioned that the use of Everolimus for the women that develop resistance to
trastuzumab has been very effective in metastatic management. The action of trustuzumab
therapy is associated with activation of the PI3K/Akt/mTOR intracellular signaling pathway.
The authors explored whether the addition of Everolimus can actively restore sensitivity to
trastuzumab in patients that have developed resistance. This randomised, double-blind,
placebo-controlled, phase 3 trial had been successful in identifying Everolimus to be effective
in significantly prolonging progression free survival in patients with trastuzumab-resistant
and taxane-pretreated, HER2-positive, advanced breast cancer. Similarly, in terms of
cardiotoxicity management, the use of pertuzumab is to be mentioned. As per Truong et al.

9HER-2 POSITIVE BREAST CANCER
(2014), pertuzumab could successfully enhance cardiac safety and avoid the risk of
asymptomatic and symptomatic left ventricular dysfunction due to much lesser cardiac
toxicity. Although, in most of the cases, the chemotherapy assisted cardiac dysfunction is
very common and it can easily affect the patients and reduce the chances of their survival. as
discussed by the Colombo et al. (2013), the use of Angiotensin-converting enzyme inhibitors
and beta-blockers have been proven to be extremely effective in managing the cardiac
dysfunction and its clinical manifestation in the cancer patients. Hence, the additional
treatment with similar drugs has also proven to be useful in managing the cardio- toxicity for
the patients suffering from HER2 positive breast cancers. Although, as the cardiac damage
ion the patients are caused by the anti-neoplastic agents, there is risk of drug-drug interaction
and other side effects for prolonged or high dose use of the cardiac medication. Hence, using
advanced and modified drugs with lesser probability of cardiac toxicity are recommended to
ensure higher survival and enhanced quality of life in this patient group (Tolaney et al. 2015).
(2014), pertuzumab could successfully enhance cardiac safety and avoid the risk of
asymptomatic and symptomatic left ventricular dysfunction due to much lesser cardiac
toxicity. Although, in most of the cases, the chemotherapy assisted cardiac dysfunction is
very common and it can easily affect the patients and reduce the chances of their survival. as
discussed by the Colombo et al. (2013), the use of Angiotensin-converting enzyme inhibitors
and beta-blockers have been proven to be extremely effective in managing the cardiac
dysfunction and its clinical manifestation in the cancer patients. Hence, the additional
treatment with similar drugs has also proven to be useful in managing the cardio- toxicity for
the patients suffering from HER2 positive breast cancers. Although, as the cardiac damage
ion the patients are caused by the anti-neoplastic agents, there is risk of drug-drug interaction
and other side effects for prolonged or high dose use of the cardiac medication. Hence, using
advanced and modified drugs with lesser probability of cardiac toxicity are recommended to
ensure higher survival and enhanced quality of life in this patient group (Tolaney et al. 2015).
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

10HER-2 POSITIVE BREAST CANCER
References:
André, F., O'Regan, R., Ozguroglu, M., Toi, M., Xu, B., Jerusalem, G., Masuda, N., Wilks,
S., Arena, F., Isaacs, C. and Yap, Y.S., 2014. Everolimus for women with trastuzumab-
resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind,
placebo-controlled phase 3 trial. The lancet oncology, 15(6), pp.580-591.
Colombo, A., Meroni, C.A., Cipolla, C.M. and Cardinale, D., 2013. Managing cardiotoxicity
of chemotherapy. Current treatment options in cardiovascular medicine, 15(4), pp.410-424.
Eschenhagen, T., Force, T., Ewer, M., de Keulenaer, G., Suter, T., Anker, S., Avkiran, M.,
de Azambuja, E., Balligand, J., Brutsaert, D., Condorelli, G., Hansen, A., Heymans, S., Hill,
J., Hirsch, E., Hilfiker-Kleiner, D., Janssens, S., de Jong, S., Neubauer, G., Pieske,
B., Ponikowski, P., Pirmohamed, M., Rauchhaus, M., Sawyer, D., Sugden, P., Wojta,
J., Zannad, F. and Shah, A. (2011). Cardiovascular side effects of cancer therapies: a position
statement from the Heart Failure Association of the European Society of
Cardiology. European Journal of Heart Failure, 13(1):1-10.
Janiszewska, M., Liu, L., Almendro, V., Kuang, Y., Paweletz, C., Sakr, R.A., Weigelt, B.,
Hanker, A.B., Chandarlapaty, S., King, T.A. and Reis-Filho, J.S., 2015. In situ single-cell
analysis identifies heterogeneity for PIK3CA mutation and HER2 amplification in HER2-
positive breast cancer. Nature genetics, 47(10), p.1212.
Onitilo, A.A., Engel, J.M. and Stankowski, R.V. (2014) Cardiovascular toxicity associated
with adjuvant trastuzumab therapy: prevalence, patient characteristics, and risk
factors, Therapeutic Advances in Drug Safety, 5(4): 154–166.
Swain, S.M., Baselga, J., Kim, S.B., Ro, J., Semiglazov, V., Campone, M., Ciruelos, E.,
Ferrero, J.M., Schneeweiss, A., Heeson, S. and Clark, E., 2015. Pertuzumab, trastuzumab,
References:
André, F., O'Regan, R., Ozguroglu, M., Toi, M., Xu, B., Jerusalem, G., Masuda, N., Wilks,
S., Arena, F., Isaacs, C. and Yap, Y.S., 2014. Everolimus for women with trastuzumab-
resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind,
placebo-controlled phase 3 trial. The lancet oncology, 15(6), pp.580-591.
Colombo, A., Meroni, C.A., Cipolla, C.M. and Cardinale, D., 2013. Managing cardiotoxicity
of chemotherapy. Current treatment options in cardiovascular medicine, 15(4), pp.410-424.
Eschenhagen, T., Force, T., Ewer, M., de Keulenaer, G., Suter, T., Anker, S., Avkiran, M.,
de Azambuja, E., Balligand, J., Brutsaert, D., Condorelli, G., Hansen, A., Heymans, S., Hill,
J., Hirsch, E., Hilfiker-Kleiner, D., Janssens, S., de Jong, S., Neubauer, G., Pieske,
B., Ponikowski, P., Pirmohamed, M., Rauchhaus, M., Sawyer, D., Sugden, P., Wojta,
J., Zannad, F. and Shah, A. (2011). Cardiovascular side effects of cancer therapies: a position
statement from the Heart Failure Association of the European Society of
Cardiology. European Journal of Heart Failure, 13(1):1-10.
Janiszewska, M., Liu, L., Almendro, V., Kuang, Y., Paweletz, C., Sakr, R.A., Weigelt, B.,
Hanker, A.B., Chandarlapaty, S., King, T.A. and Reis-Filho, J.S., 2015. In situ single-cell
analysis identifies heterogeneity for PIK3CA mutation and HER2 amplification in HER2-
positive breast cancer. Nature genetics, 47(10), p.1212.
Onitilo, A.A., Engel, J.M. and Stankowski, R.V. (2014) Cardiovascular toxicity associated
with adjuvant trastuzumab therapy: prevalence, patient characteristics, and risk
factors, Therapeutic Advances in Drug Safety, 5(4): 154–166.
Swain, S.M., Baselga, J., Kim, S.B., Ro, J., Semiglazov, V., Campone, M., Ciruelos, E.,
Ferrero, J.M., Schneeweiss, A., Heeson, S. and Clark, E., 2015. Pertuzumab, trastuzumab,

11HER-2 POSITIVE BREAST CANCER
and docetaxel in HER2-positive metastatic breast cancer. New England Journal of
Medicine, 372(8), pp.724-734.
Tolaney, S.M., Barry, W.T., Dang, C.T., Yardley, D.A., Moy, B., Marcom, P.K., Albain,
K.S., Rugo, H.S., Ellis, M., Shapira, I. and Wolff, A.C., 2015. Adjuvant paclitaxel and
trastuzumab for node-negative, HER2-positive breast cancer. New England Journal of
Medicine, 372(2), pp.134-141.
Truong, J., Yan, A.T., Cramarossa, G. and Chan, K.K., 2014. Chemotherapy-induced
cardiotoxicity: detection, prevention, and management. Canadian Journal of
Cardiology, 30(8), pp.869-878.
and docetaxel in HER2-positive metastatic breast cancer. New England Journal of
Medicine, 372(8), pp.724-734.
Tolaney, S.M., Barry, W.T., Dang, C.T., Yardley, D.A., Moy, B., Marcom, P.K., Albain,
K.S., Rugo, H.S., Ellis, M., Shapira, I. and Wolff, A.C., 2015. Adjuvant paclitaxel and
trastuzumab for node-negative, HER2-positive breast cancer. New England Journal of
Medicine, 372(2), pp.134-141.
Truong, J., Yan, A.T., Cramarossa, G. and Chan, K.K., 2014. Chemotherapy-induced
cardiotoxicity: detection, prevention, and management. Canadian Journal of
Cardiology, 30(8), pp.869-878.

12HER-2 POSITIVE BREAST CANCER
Part C
Uropathogenic Escherichia coli or (UPEC) infections are potent causes of community
acquired infections that lead to urinary tract infections and bladder infections and often have
recurrent episodes. The treatment for UPOEC infections should be planned by keeping in
mind the virulence factors that cause the infection. The UPEC infections are associated with a
myriad of virulence factors; the pertinent virulence factors include the structural surface
components like the lipopolysaccharides of the cell membrane, polysacharride capsules,
flagella and pili structures, proteins of the outer membrane, non-pillus adhesions, secretory
toxins and siderophore and iron-uptake receptors (Terlizzi et al. 2017). The aerobactin
system, hemolysins and resistance of the pathogenic bacteria to serum killing are included as
virulence factors. These virulence factors play a crucial role in urinary tract and bladder
infection pathogenesis. The capacity of the virulent factors and causal agents to adhere to the
host cells in urinary epithelium underlies the important criteria of determinant for
pathogenicity. UPEC infection is accompanied with colonisation of microbes in the
periurethtral and urethral regions, biofilm formation leading to urinary tract infections. The
bacterial components called curli secrete protein components with amyloid firbrillar structure
and characteristics. These amyloid fibrils have a crucial role in formation of biofilm (Asadi et
al. 2014). UPEC adheres to the urinary bladder in type-1 pili dependent mechanism. The
group of adherent bacteria undergo internalisation into the apical membrane epithelial cells.
UPEDC causes neutralisation of the lysosomes, as is sensed by lysosomal membrane protein.
This causes activation of exocytosis pathways. The UPEC internalisation activates toll like
receptor 4 (TLR 4). This in turn causes ubiquitination of TRAF-3 (TNF receptor associated
factor 3). This is directly correlated with exocytosis complex formation (Al-Badr and Al-
Part C
Uropathogenic Escherichia coli or (UPEC) infections are potent causes of community
acquired infections that lead to urinary tract infections and bladder infections and often have
recurrent episodes. The treatment for UPOEC infections should be planned by keeping in
mind the virulence factors that cause the infection. The UPEC infections are associated with a
myriad of virulence factors; the pertinent virulence factors include the structural surface
components like the lipopolysaccharides of the cell membrane, polysacharride capsules,
flagella and pili structures, proteins of the outer membrane, non-pillus adhesions, secretory
toxins and siderophore and iron-uptake receptors (Terlizzi et al. 2017). The aerobactin
system, hemolysins and resistance of the pathogenic bacteria to serum killing are included as
virulence factors. These virulence factors play a crucial role in urinary tract and bladder
infection pathogenesis. The capacity of the virulent factors and causal agents to adhere to the
host cells in urinary epithelium underlies the important criteria of determinant for
pathogenicity. UPEC infection is accompanied with colonisation of microbes in the
periurethtral and urethral regions, biofilm formation leading to urinary tract infections. The
bacterial components called curli secrete protein components with amyloid firbrillar structure
and characteristics. These amyloid fibrils have a crucial role in formation of biofilm (Asadi et
al. 2014). UPEC adheres to the urinary bladder in type-1 pili dependent mechanism. The
group of adherent bacteria undergo internalisation into the apical membrane epithelial cells.
UPEDC causes neutralisation of the lysosomes, as is sensed by lysosomal membrane protein.
This causes activation of exocytosis pathways. The UPEC internalisation activates toll like
receptor 4 (TLR 4). This in turn causes ubiquitination of TRAF-3 (TNF receptor associated
factor 3). This is directly correlated with exocytosis complex formation (Al-Badr and Al-
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

13HER-2 POSITIVE BREAST CANCER
Shaikh 2013). UPEC still can gain entry into the bladder epithelium and establish
intracellular bacterial biofilm formation.
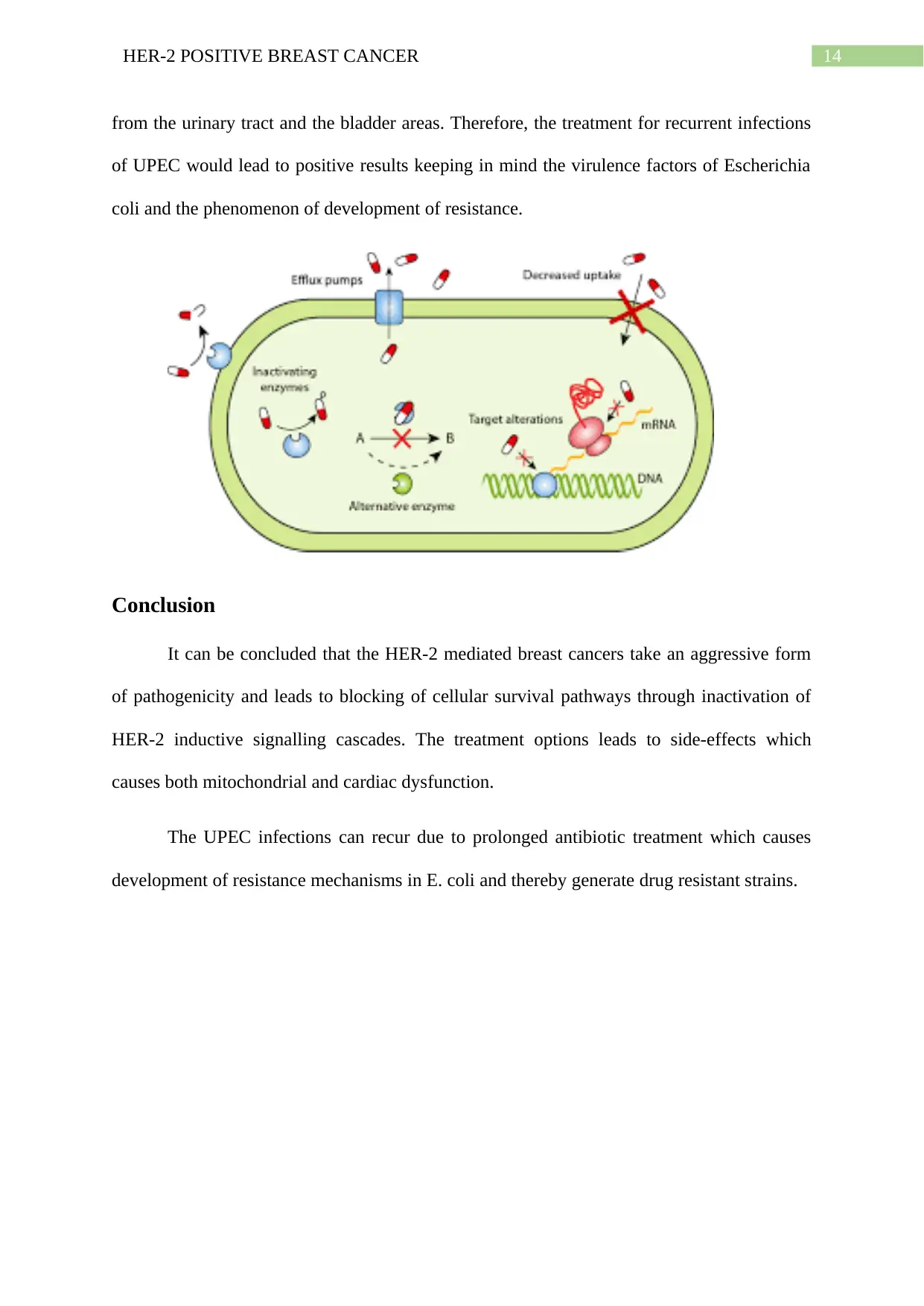
The increasing UPEC infections are emergent due to development of drug resistance
strains. The mechanisms of drug resistance development in UPEC involve inhibition of the
hydrolytic enzymes by the beta-lactamases, lack of inactivation by aminoglycoside acetyl
transferase, the alteration of permeability through the use of efflux pumps, target site
inactivation and inactivation acquired through horizontal gene transfer methods (Flores-
Mireles et al.2015). Insertion of genetic elements include insertion sequences, transposons,
integrons and gene cassettes. The integrons encode gene sequences expressing proteins which
result in development of antibiotic resistance. The integrons are carried within transposons.
Prolonged urinary tract infections can lead to recurrence of UPEC infections; the
failure of treatment and diagnosis can lead to recurrent episodes. The potential risk factors
need to be assessed in order to provide a line of treatment. Treatment involves the oral
administration of prophylactic and antibiotics to the UPEC infected individuals. The
continued long-term administration of antibiotics leads to development of resistance in the
causative bacteria. The components of antibiotic treatment involves a combination of
antifolate components; trimethoprim and sulfamethoxazole. This combination reduces the
recurrence of UTI infect ions; however, routine and prolonged treatment of this combination
of antibiotics develops resistance in E. coli. E.coli develops resistance through horizontal
gene transfer mediated by integrons and transposons (Al-Badr and Al-Shaikh 2013). It can be
therefore said that, the UPEC infect ions can be cured through antibiotics; however,
prolonged reliance on antibiotics are the cause of UPEC recurrence. Recurrent infections are
mainly caused by antibiotic-resistant forms of bacteria. The development of antibiotic
resistance in bacteria through prolonged antibiotic use poses a treatment threat, therefore the
continued antibiotic treatment results in failure and cannot eliminate the bacterial pathogens
Shaikh 2013). UPEC still can gain entry into the bladder epithelium and establish
intracellular bacterial biofilm formation.
The increasing UPEC infections are emergent due to development of drug resistance
strains. The mechanisms of drug resistance development in UPEC involve inhibition of the
hydrolytic enzymes by the beta-lactamases, lack of inactivation by aminoglycoside acetyl
transferase, the alteration of permeability through the use of efflux pumps, target site
inactivation and inactivation acquired through horizontal gene transfer methods (Flores-
Mireles et al.2015). Insertion of genetic elements include insertion sequences, transposons,
integrons and gene cassettes. The integrons encode gene sequences expressing proteins which
result in development of antibiotic resistance. The integrons are carried within transposons.
Prolonged urinary tract infections can lead to recurrence of UPEC infections; the
failure of treatment and diagnosis can lead to recurrent episodes. The potential risk factors
need to be assessed in order to provide a line of treatment. Treatment involves the oral
administration of prophylactic and antibiotics to the UPEC infected individuals. The
continued long-term administration of antibiotics leads to development of resistance in the
causative bacteria. The components of antibiotic treatment involves a combination of
antifolate components; trimethoprim and sulfamethoxazole. This combination reduces the
recurrence of UTI infect ions; however, routine and prolonged treatment of this combination
of antibiotics develops resistance in E. coli. E.coli develops resistance through horizontal
gene transfer mediated by integrons and transposons (Al-Badr and Al-Shaikh 2013). It can be
therefore said that, the UPEC infect ions can be cured through antibiotics; however,
prolonged reliance on antibiotics are the cause of UPEC recurrence. Recurrent infections are
mainly caused by antibiotic-resistant forms of bacteria. The development of antibiotic
resistance in bacteria through prolonged antibiotic use poses a treatment threat, therefore the
continued antibiotic treatment results in failure and cannot eliminate the bacterial pathogens
14HER-2 POSITIVE BREAST CANCER
from the urinary tract and the bladder areas. Therefore, the treatment for recurrent infections
of UPEC would lead to positive results keeping in mind the virulence factors of Escherichia
coli and the phenomenon of development of resistance.
Conclusion
It can be concluded that the HER-2 mediated breast cancers take an aggressive form
of pathogenicity and leads to blocking of cellular survival pathways through inactivation of
HER-2 inductive signalling cascades. The treatment options leads to side-effects which
causes both mitochondrial and cardiac dysfunction.
The UPEC infections can recur due to prolonged antibiotic treatment which causes
development of resistance mechanisms in E. coli and thereby generate drug resistant strains.
from the urinary tract and the bladder areas. Therefore, the treatment for recurrent infections
of UPEC would lead to positive results keeping in mind the virulence factors of Escherichia
coli and the phenomenon of development of resistance.
Conclusion
It can be concluded that the HER-2 mediated breast cancers take an aggressive form
of pathogenicity and leads to blocking of cellular survival pathways through inactivation of
HER-2 inductive signalling cascades. The treatment options leads to side-effects which
causes both mitochondrial and cardiac dysfunction.
The UPEC infections can recur due to prolonged antibiotic treatment which causes
development of resistance mechanisms in E. coli and thereby generate drug resistant strains.

15HER-2 POSITIVE BREAST CANCER
References
Al-Badr, A. and Al-Shaikh, G., 2013. Recurrent urinary tract infections management in
women: a review. Sultan Qaboos University Medical Journal, 13(3), p.359.
Asadi, S., Kargar, M., Solhjoo, K., Najafi, A. and Ghorbani-Dalini, S., 2014. The association
of virulence determinants of uropathogenic Escherichia coli with antibiotic
resistance. Jundishapur journal of microbiology, 7(5).
Flores-Mireles, A.L., Walker, J.N., Caparon, M. and Hultgren, S.J., 2015. Urinary tract
infections: epidemiology, mechanisms of infection and treatment options. Nature reviews
microbiology, 13(5), p.269.
Terlizzi, M.E., Gribaudo, G. and Maffei, M.E., 2017. UroPathogenic Escherichia coli
(UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic
antimicrobial strategies. Frontiers in microbiology, 8, p.1566.
References
Al-Badr, A. and Al-Shaikh, G., 2013. Recurrent urinary tract infections management in
women: a review. Sultan Qaboos University Medical Journal, 13(3), p.359.
Asadi, S., Kargar, M., Solhjoo, K., Najafi, A. and Ghorbani-Dalini, S., 2014. The association
of virulence determinants of uropathogenic Escherichia coli with antibiotic
resistance. Jundishapur journal of microbiology, 7(5).
Flores-Mireles, A.L., Walker, J.N., Caparon, M. and Hultgren, S.J., 2015. Urinary tract
infections: epidemiology, mechanisms of infection and treatment options. Nature reviews
microbiology, 13(5), p.269.
Terlizzi, M.E., Gribaudo, G. and Maffei, M.E., 2017. UroPathogenic Escherichia coli
(UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic
antimicrobial strategies. Frontiers in microbiology, 8, p.1566.
1 out of 16
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.





