Practical Assignment: Preparing Solutions, Dilutions, and Absorbance
VerifiedAdded on 2022/05/30
|8
|2091
|42
Practical Assignment
AI Summary
This practical assignment focuses on the fundamental techniques of preparing solutions and performing dilutions, essential skills in an analytical biochemistry lab. The assignment covers the preparation of stock solutions, calculation of solute amounts, and the use of appropriate glassware and tools. It then delves into two primary dilution methods: linear and serial dilutions, with detailed explanations and examples. Students are guided through the preparation of a bromophenol blue stock solution and subsequent working solutions using both serial and linear dilution methods. The assignment also includes the measurement of absorbance of the prepared working solutions using a spectrophotometer, emphasizing the relationship between concentration and absorbance. The document incorporates calculations, procedures, and observations, providing a complete understanding of solution preparation and dilution techniques. The practical concludes with questions to assess the student's understanding of the concepts and the observed results.

PRACTICAL 2: PREPARING SOLUTIONS AND MAKING DILUTIONS
INTRODUCTION
Preparing solutions is a very common activity in an analytical biochemistry lab. Proper
solution preparation requires basic math skills, accurate measurement using
appropriate and suitable tools or glassware, and the ability to follow instructions. A
solution is a homogenous mixture of “solute” dissolved in bulk liquid known as
“solvent”. Solutions can be described by their solute concentration, a measure of how
much solute is present per unit of solution. A concentrated solution is generally called
a “stock solution,” and the diluted solution is called the “working solution”. Preparing
a concentrated stock solution saves a lot of time. It is easier to store stock solution
than a large volume of diluted working solutions. Making a working solution simply
requires diluting some volume of stock solution to the concentration needed. Every
biochemistry student must learn and know how to prepare a stock solution and
making a working solution.
OBJECTIVES
1. To prepare a stock solution.
2. To prepare a working solution by diluting a stock solution using linear and serial
dilution methods.
3. To measure the absorbance of working solutions at various concentrations using
a spectrophotometer.
APPARATUS
1. 10-mL beaker
2. 100-mL beaker
3. Test tubes
4. Graduated cylinder
5. UV Spectrophotometer cuvette
6. Pipette
7. Spatula
8. Dispensing bottle with distilled water
9. Distilled water
10. Pipette bulb/Pipette pump
11. Test tube rack
12. Weighing paper
12 | P a g e
INTRODUCTION
Preparing solutions is a very common activity in an analytical biochemistry lab. Proper
solution preparation requires basic math skills, accurate measurement using
appropriate and suitable tools or glassware, and the ability to follow instructions. A
solution is a homogenous mixture of “solute” dissolved in bulk liquid known as
“solvent”. Solutions can be described by their solute concentration, a measure of how
much solute is present per unit of solution. A concentrated solution is generally called
a “stock solution,” and the diluted solution is called the “working solution”. Preparing
a concentrated stock solution saves a lot of time. It is easier to store stock solution
than a large volume of diluted working solutions. Making a working solution simply
requires diluting some volume of stock solution to the concentration needed. Every
biochemistry student must learn and know how to prepare a stock solution and
making a working solution.
OBJECTIVES
1. To prepare a stock solution.
2. To prepare a working solution by diluting a stock solution using linear and serial
dilution methods.
3. To measure the absorbance of working solutions at various concentrations using
a spectrophotometer.
APPARATUS
1. 10-mL beaker
2. 100-mL beaker
3. Test tubes
4. Graduated cylinder
5. UV Spectrophotometer cuvette
6. Pipette
7. Spatula
8. Dispensing bottle with distilled water
9. Distilled water
10. Pipette bulb/Pipette pump
11. Test tube rack
12. Weighing paper
12 | P a g e
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
13. UV-Vis Spectrophotometer
14. Analytical balance
Chemical: Bromophenol Blue
Lab Activity 1: Preparing solutions
Before preparing a solution, you must be able to identify or recognize the following
questions; (i) what is the substance or chemical to be dissolved?, (ii) what is the
amount required?, (iii) what is the most suitable solvent to be used?, (iv) how to
calculate these amount, and, (v) what tools or glassware should be used to obtain
accurate measurements and right concentration. The amount of substance is often
measured as weight, i.e. in mg or g or kg. Mole is also used representing the amount
of substance, the mass of a mole of molecules if the formula weight is in grams. As an
example, the molecular mass of amino acid glycine is 75.07 g/mol or sometime in
‘Dalton’ unit, i.e. 75 Da.
Concentration is the amount divided by the volume it is dissolved or dispersed in. It is
possible to use different units to express concentration depending on the states of
matter (i.e. solid, liquid or gas) that the two substances are in. Typically,
concentrations are measured as the mass of solute per unit volume of solution, i.e.,
A. Molar solutions (Unit = M= moles/L)
A 1.0 molar (1.0 M) solution is equivalent to 1.0 mole (formula weight [FW] [g/mole])
of solute dissolved in 1.0 L of solvent.
Example 1: To prepare a litre of 0.15 M solution from a dry reagent with FW of 194.0
g/mole, amount of solute required is 194.0 g/mole x 0.15 mole/L = 29.1 g/L
B. Percent solution (= parts per hundred)
1. In most cases, reagents are prepared as percent concentrations, a dry chemical is
mixed as dry mass (g) per volume (g/100 mL), [unit (w/v)].
Example 2: To prepare a 10% (w/v) NaCl solution, 10 g NaCl is dissolved in 100 mL
of solvent
Example 3: To prepare 150 mL of 6% (w/v) reagent A, 6 g/100 mL x 150 mL = 6 g
x 1.5 = 9 g of A is dissolved in 150 mL solvent
13 | P a g e
14. Analytical balance
Chemical: Bromophenol Blue
Lab Activity 1: Preparing solutions
Before preparing a solution, you must be able to identify or recognize the following
questions; (i) what is the substance or chemical to be dissolved?, (ii) what is the
amount required?, (iii) what is the most suitable solvent to be used?, (iv) how to
calculate these amount, and, (v) what tools or glassware should be used to obtain
accurate measurements and right concentration. The amount of substance is often
measured as weight, i.e. in mg or g or kg. Mole is also used representing the amount
of substance, the mass of a mole of molecules if the formula weight is in grams. As an
example, the molecular mass of amino acid glycine is 75.07 g/mol or sometime in
‘Dalton’ unit, i.e. 75 Da.
Concentration is the amount divided by the volume it is dissolved or dispersed in. It is
possible to use different units to express concentration depending on the states of
matter (i.e. solid, liquid or gas) that the two substances are in. Typically,
concentrations are measured as the mass of solute per unit volume of solution, i.e.,
A. Molar solutions (Unit = M= moles/L)
A 1.0 molar (1.0 M) solution is equivalent to 1.0 mole (formula weight [FW] [g/mole])
of solute dissolved in 1.0 L of solvent.
Example 1: To prepare a litre of 0.15 M solution from a dry reagent with FW of 194.0
g/mole, amount of solute required is 194.0 g/mole x 0.15 mole/L = 29.1 g/L
B. Percent solution (= parts per hundred)
1. In most cases, reagents are prepared as percent concentrations, a dry chemical is
mixed as dry mass (g) per volume (g/100 mL), [unit (w/v)].
Example 2: To prepare a 10% (w/v) NaCl solution, 10 g NaCl is dissolved in 100 mL
of solvent
Example 3: To prepare 150 mL of 6% (w/v) reagent A, 6 g/100 mL x 150 mL = 6 g
x 1.5 = 9 g of A is dissolved in 150 mL solvent
13 | P a g e

2. If using liquid reagents, the percent concentration is based upon volume per
volume (mL/100 mL), [unit (v/v)].
Example 4: To prepare 75% (v/v) ethanol, mix 75 mL of ethanol with 25 mL water,
making up the volume to 100 mL
3. Concentration may also be expressed as percent of dry mass per mass of solvent
(g/100 g), [unit w/w)].
Example 5: To prepare a 20% (w/w) of Triton X-100, 20 g of Triton X-100 is mixed
with 80 g solvent
Example 6: To prepare a 200 g of 20% (w/w) reagent B, 20 g/100 g x 200 g = 20 g
x 2 = 40 g of B is mixed with 160 g solvent
C. Conversion from % to molarity and from molarity to %
To convert from percent concentration solution to molarity, use the formula below.
Example 7: To convert a 6% solution of C with FW of 325 g/mole to molarity value,
[6 g/100 mL) x 10]/325 g/mole = 0.2 M
To convert from molarity to percent concentration solution, use the formula below.
Example 8: To convert a 0.06 M solution of a chemical having FW of 178 g/mole to
percent solution,
[0.06 mole/L] x 178 g/mol /10 = 1.06% solution
Exercise 1: Preparation of a bromophenol blue stock solution
1. Prepare 50 mL bromophenol blue stock solution at concentration of 0.1 mg/mL.
Use distilled water as solvent. What is the amount of bromophenol blue powder
required? Show your calculation. Keep the solution for Exercise 2.
14 | P a g e
volume (mL/100 mL), [unit (v/v)].
Example 4: To prepare 75% (v/v) ethanol, mix 75 mL of ethanol with 25 mL water,
making up the volume to 100 mL
3. Concentration may also be expressed as percent of dry mass per mass of solvent
(g/100 g), [unit w/w)].
Example 5: To prepare a 20% (w/w) of Triton X-100, 20 g of Triton X-100 is mixed
with 80 g solvent
Example 6: To prepare a 200 g of 20% (w/w) reagent B, 20 g/100 g x 200 g = 20 g
x 2 = 40 g of B is mixed with 160 g solvent
C. Conversion from % to molarity and from molarity to %
To convert from percent concentration solution to molarity, use the formula below.
Example 7: To convert a 6% solution of C with FW of 325 g/mole to molarity value,
[6 g/100 mL) x 10]/325 g/mole = 0.2 M
To convert from molarity to percent concentration solution, use the formula below.
Example 8: To convert a 0.06 M solution of a chemical having FW of 178 g/mole to
percent solution,
[0.06 mole/L] x 178 g/mol /10 = 1.06% solution
Exercise 1: Preparation of a bromophenol blue stock solution
1. Prepare 50 mL bromophenol blue stock solution at concentration of 0.1 mg/mL.
Use distilled water as solvent. What is the amount of bromophenol blue powder
required? Show your calculation. Keep the solution for Exercise 2.
14 | P a g e
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

2. Given the FW of bromophenol blue is 670 g/mole. Convert the concentration to
molarity and percent (w/v) of your stock solution. Show your calculation.
3. What have you learnt from this lab activity?
Lab Activity 2: Making dilutions
Dilution refers to the process of adding additional solvent to a solution to decrease its
concentration. Usually, a stock solution is prepared first which has the highest
concentration. Dilution is done from the stock solution to get a series of working
solutions that contain a lower concentration than the stock solution. This can be done
in many ways.
A. Dilution factor method
To do a dilution using the dilution factor method, a unit volume of a solution or liquid is
combined with an appropriate volume or solvent to obtain the desired concentration.
The dilution factor is the total number of unit volumes, where material will be
dissolved.
Example 9: A 1 to 5 dilution or 1:5 dilution entails containing 1 unit volume of stock +
4 unit volumes of solvent, thus 1 + 4 = 5 = dilution factor
Question:
Explain how to prepare a 1X solution of saline sodium sulphate (SSC) from a stock
solution of 20X SSC.
15 | P a g e
molarity and percent (w/v) of your stock solution. Show your calculation.
3. What have you learnt from this lab activity?
Lab Activity 2: Making dilutions
Dilution refers to the process of adding additional solvent to a solution to decrease its
concentration. Usually, a stock solution is prepared first which has the highest
concentration. Dilution is done from the stock solution to get a series of working
solutions that contain a lower concentration than the stock solution. This can be done
in many ways.
A. Dilution factor method
To do a dilution using the dilution factor method, a unit volume of a solution or liquid is
combined with an appropriate volume or solvent to obtain the desired concentration.
The dilution factor is the total number of unit volumes, where material will be
dissolved.
Example 9: A 1 to 5 dilution or 1:5 dilution entails containing 1 unit volume of stock +
4 unit volumes of solvent, thus 1 + 4 = 5 = dilution factor
Question:
Explain how to prepare a 1X solution of saline sodium sulphate (SSC) from a stock
solution of 20X SSC.
15 | P a g e
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
B. Mixing parts or volumes
Mixing parts of volumes is different than the simple dilution. DO NOT BE CONFUSED! If
you are instructed to make up 1:3 acetic ethanol solution, you probably mix one unit
volume of acetic acid and three unit volume of ethanol. If you are requested to make a
1:3 dilution of acetic ethanol, you would mix one unit volume of acetic acid with two
volumes of ethanol.
Question:
You are requested to prepare a solution containing a ratio of 1:9:0.5 of water, ethanol
and acetic acid. How would you prepare it?
C. Serial dilution
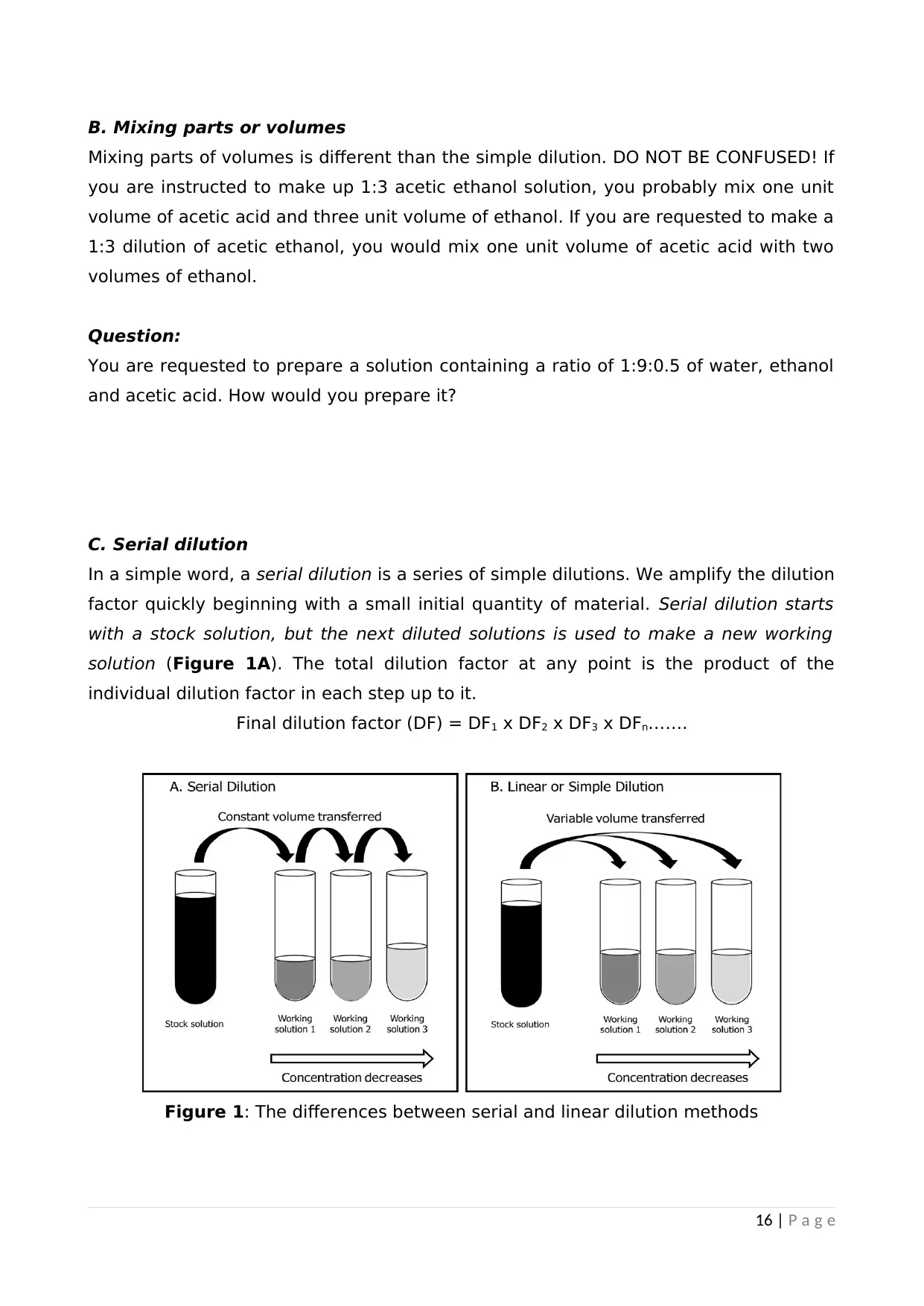
In a simple word, a serial dilution is a series of simple dilutions. We amplify the dilution
factor quickly beginning with a small initial quantity of material. Serial dilution starts
with a stock solution, but the next diluted solutions is used to make a new working
solution (Figure 1A). The total dilution factor at any point is the product of the
individual dilution factor in each step up to it.
Final dilution factor (DF) = DF1 x DF2 x DF3 x DFn…….
Figure 1: The differences between serial and linear dilution methods
16 | P a g e
Mixing parts of volumes is different than the simple dilution. DO NOT BE CONFUSED! If
you are instructed to make up 1:3 acetic ethanol solution, you probably mix one unit
volume of acetic acid and three unit volume of ethanol. If you are requested to make a
1:3 dilution of acetic ethanol, you would mix one unit volume of acetic acid with two
volumes of ethanol.
Question:
You are requested to prepare a solution containing a ratio of 1:9:0.5 of water, ethanol
and acetic acid. How would you prepare it?
C. Serial dilution
In a simple word, a serial dilution is a series of simple dilutions. We amplify the dilution
factor quickly beginning with a small initial quantity of material. Serial dilution starts
with a stock solution, but the next diluted solutions is used to make a new working
solution (Figure 1A). The total dilution factor at any point is the product of the
individual dilution factor in each step up to it.
Final dilution factor (DF) = DF1 x DF2 x DF3 x DFn…….
Figure 1: The differences between serial and linear dilution methods
16 | P a g e
Example 10: In microbiology class, students perform a three step 1:100 serial dilution
of a bacterial culture. The initial step, 1 unit of volume culture (10 μL) with 99 unit
volumes of broth (990 μL) = 1:100 dilution. In the second step, one volume of “the
1:100 dilution” is combined with 99 unit volumes of broth, the total dilution of current
solution is 1:(100 x 100) = 1: 10,000. And the final or total dilution would be 1:(100 X
10,000) = 1: 1,000,000. The bacteria concentration is now one million times less than
in the original sample.
Question:
What is the final concentration of your bromophenol blue (from Exercise 1), if we
perform a three steps of 1:100 serial dilution each?
Exercise 2A: Preparing solutions using serial dilution
Use your 0.1 mg/mL bromophenol blue stock solution (previously prepared in
Exercise 1) to prepare a series of 10 mL working solutions. Write down the volume of
stock solution and distilled water used to make the working solutions according to
dilution factor stated in Table-1. Prepare at least two replicates for each
concentration. Keep your solution for Exercise 3.
Table-1: A serial dilution of bromophenol blue stock solution.
Dilution
factor
(10X-serial)
Volume of
solution
added from
the previous
test tube
(mL)
Amount of distilled
water added to 10
mL final volume,
(mL)
Final
concentration of
bromophenol blue
(mg/mL)
0
10
100
1,000
10,000
100,000
D. Linear dilution or making fixed volume of specific concentration
Linear or simple dilution (Figure 1B) is very often used to prepare a specific volume
of known concentration due to limited availability of liquid materials or to reduce the
quantity of waste. To prepare a series of working solutions using a linear dilution
17 | P a g e
of a bacterial culture. The initial step, 1 unit of volume culture (10 μL) with 99 unit
volumes of broth (990 μL) = 1:100 dilution. In the second step, one volume of “the
1:100 dilution” is combined with 99 unit volumes of broth, the total dilution of current
solution is 1:(100 x 100) = 1: 10,000. And the final or total dilution would be 1:(100 X
10,000) = 1: 1,000,000. The bacteria concentration is now one million times less than
in the original sample.
Question:
What is the final concentration of your bromophenol blue (from Exercise 1), if we
perform a three steps of 1:100 serial dilution each?
Exercise 2A: Preparing solutions using serial dilution
Use your 0.1 mg/mL bromophenol blue stock solution (previously prepared in
Exercise 1) to prepare a series of 10 mL working solutions. Write down the volume of
stock solution and distilled water used to make the working solutions according to
dilution factor stated in Table-1. Prepare at least two replicates for each
concentration. Keep your solution for Exercise 3.
Table-1: A serial dilution of bromophenol blue stock solution.
Dilution
factor
(10X-serial)
Volume of
solution
added from
the previous
test tube
(mL)
Amount of distilled
water added to 10
mL final volume,
(mL)
Final
concentration of
bromophenol blue
(mg/mL)
0
10
100
1,000
10,000
100,000
D. Linear dilution or making fixed volume of specific concentration
Linear or simple dilution (Figure 1B) is very often used to prepare a specific volume
of known concentration due to limited availability of liquid materials or to reduce the
quantity of waste. To prepare a series of working solutions using a linear dilution
17 | P a g e
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
method, the source of solutes to make all the working solutions comes from the stock
solution.
A simple equation below is a quick approach to calculating such dilutions:
M = concentration, V = volume
(Source solution attributes) M1V1 = M2V2 (new solution attributes)
This equation is also applicable to the serial dilution method. To make a dilute solution
without calculating the concentrations, you can rely on dilution factor as follows:
Dilution factor (d.f.) = Final volume / Initial volume
Exercise 2B: Preparing solutions using linear dilution
1. Use the bromophenol blue stock solution previously prepared in Exercise 1.
2. Choose a range of concentration for your bromophenol blue working solutions.
Prepare the solutions using the linear dilution method. Remember to have
replicates for each concentration.
3. Your final volume for each concentration should be 10 mL.
4. Write down the volume of stock and distilled water required to prepare the
solutions into Table-2. Keep your solution for Exercise 3.
Table-2: A linear dilution of bromophenol blue stock solution
Test
tube
Volume of
stock solution
(mL)
Volume of
distilled
water (mL)
Final concentration
of bromophenol blue
(mg/mL)
1. 0 10 0
2.
3.
4.
5.
6.
Exercise 3: Relationship between concentration and absorbance
1. Set the spectrophotometer to the maximum wavelength of bromophenol blue you
obtained in Practical 1.
2. Read the absorbance for 10x, 1,000x and 100,000x in Table-1 and test tubes
2,4 and 6 in Table-2.
3. Fill in the absorbance obtained in Table-3 below:
18 | P a g e
solution.
A simple equation below is a quick approach to calculating such dilutions:
M = concentration, V = volume
(Source solution attributes) M1V1 = M2V2 (new solution attributes)
This equation is also applicable to the serial dilution method. To make a dilute solution
without calculating the concentrations, you can rely on dilution factor as follows:
Dilution factor (d.f.) = Final volume / Initial volume
Exercise 2B: Preparing solutions using linear dilution
1. Use the bromophenol blue stock solution previously prepared in Exercise 1.
2. Choose a range of concentration for your bromophenol blue working solutions.
Prepare the solutions using the linear dilution method. Remember to have
replicates for each concentration.
3. Your final volume for each concentration should be 10 mL.
4. Write down the volume of stock and distilled water required to prepare the
solutions into Table-2. Keep your solution for Exercise 3.
Table-2: A linear dilution of bromophenol blue stock solution
Test
tube
Volume of
stock solution
(mL)
Volume of
distilled
water (mL)
Final concentration
of bromophenol blue
(mg/mL)
1. 0 10 0
2.
3.
4.
5.
6.
Exercise 3: Relationship between concentration and absorbance
1. Set the spectrophotometer to the maximum wavelength of bromophenol blue you
obtained in Practical 1.
2. Read the absorbance for 10x, 1,000x and 100,000x in Table-1 and test tubes
2,4 and 6 in Table-2.
3. Fill in the absorbance obtained in Table-3 below:
18 | P a g e
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Table-3: Absorbance of bromophenol blue at various concentrations prepared
using serial and linear dilutions
Test
tube
Serial Dilution Linear Dilution
[Bromophenol
blue]
(mg/mL)
Absorbance
(= ??? nm)
[Bromophenol
blue]
(mg/mL)
Absorbance
(= ??? nm)
1.
2.
3.
Questions:
1. What did you observe from the data obtained in Table-3?
2. What can you conclude from this exercise?
19 | P a g e
using serial and linear dilutions
Test
tube
Serial Dilution Linear Dilution
[Bromophenol
blue]
(mg/mL)
Absorbance
(= ??? nm)
[Bromophenol
blue]
(mg/mL)
Absorbance
(= ??? nm)
1.
2.
3.
Questions:
1. What did you observe from the data obtained in Table-3?
2. What can you conclude from this exercise?
19 | P a g e
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





