LACTOFERRIN Medical Question and Answer 2022
VerifiedAdded on 2022/09/15
|12
|2059
|22
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: MEDICAL
LACTOFERRIN
Name of the Student
Name of the University
Author Note
LACTOFERRIN
Name of the Student
Name of the University
Author Note
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

1MEDICAL
Table of Contents
Answer 1....................................................................................................................................2
Answer 2....................................................................................................................................2
ProtScale..................................................................................................................................4
User-provided sequence:...................................................................................................4
Answer 3....................................................................................................................................6
Answer 4....................................................................................................................................7
Bibliography...............................................................................................................................9
Table of Contents
Answer 1....................................................................................................................................2
Answer 2....................................................................................................................................2
ProtScale..................................................................................................................................4
User-provided sequence:...................................................................................................4
Answer 3....................................................................................................................................6
Answer 4....................................................................................................................................7
Bibliography...............................................................................................................................9

2MEDICAL
Introduction
Lactoferrin has been defined as a multifunctional, globular glycoprotein that has been widely
found inside secretory fluids including tears, milk and saliva. Human colostrum has been
found to contain the highest concentration of lactoferrin. This can be purified from milk by
the use of various chromatographic techniques. This paper will throw a light on the amino
acid composition of lactoferrin including its primary structure and the past and present
purification steps.
Answer 1
Primary structure of Lactoferrin with domains and subdomains, and disulfide
connections with glycosylated amino acids if present:
N-terminal end-
MKLVFLVLLFLGALGLCLAGRRRRSVQWCAVSQPEATKCFQWQRNMRRVRGPPVS
CIKRDSPIQCIQAIAENRADAVTLDGGFIYEAGLAPYKLRPVAAEVYGTERQPRTHYY
AVAVVKKGGSFQLNELQGLKSCHTGLRRNAGWNVPIGTLRPFLNWTGPPEPIAVAR
FFSASCVPGADKGQFPNLCRLCAGTGENKCAFSSQEPYFSYSGAFKCLRDGADVAFI
RESTVFEDLSDEAERDEYELLCPDNTRKPVDKFKDCHLARVPSHAVVARSVNGKED
AIWNLLRQAQEKFGKDKSPKFQLFGSPSGQKDLLFKDSAIGFSRVPPRIDSGLYLGSG
YFTAIQNLRKSEEEVAARRARVVWCAVGEQELRKCNQWSGLSEGSVTCSSASTTED
CIALVLKGEADAMSLDGGYVYTAGKCGLVPVLAENYKSQQSSDPDPNCVDRVEGY
LAVAVVRRSDTSLTWNSVKGKKSCHTAVDRTAGWNIPMGLLFNQTGSCKFDEYFS
QSCAPGSDPRSNLCALCIGDEQGENKCVPNSNERYYGYTGAFRCLAEDAGDAFVKG
VTVLQNTDGNNNEAWAKDLKLADFALLCLDGKRKPVTEARSCHLAMAPNHAVVS
RMDKVERLKQVLLHQQAKFGRNGSDCPDKFCLFQSETKNLLFNDNTECLARLHGKT
TYEKYLGPQYVAGITNLKKCSTS PLLEACEFL K- C terminal end
Yellow domain= Transferrin like domain 1H
Blue domain= Transferring like domain 2
No glycosylation or disulphide bridges have been observed in the primary structure of
lactoferrin.
Introduction
Lactoferrin has been defined as a multifunctional, globular glycoprotein that has been widely
found inside secretory fluids including tears, milk and saliva. Human colostrum has been
found to contain the highest concentration of lactoferrin. This can be purified from milk by
the use of various chromatographic techniques. This paper will throw a light on the amino
acid composition of lactoferrin including its primary structure and the past and present
purification steps.
Answer 1
Primary structure of Lactoferrin with domains and subdomains, and disulfide
connections with glycosylated amino acids if present:
N-terminal end-
MKLVFLVLLFLGALGLCLAGRRRRSVQWCAVSQPEATKCFQWQRNMRRVRGPPVS
CIKRDSPIQCIQAIAENRADAVTLDGGFIYEAGLAPYKLRPVAAEVYGTERQPRTHYY
AVAVVKKGGSFQLNELQGLKSCHTGLRRNAGWNVPIGTLRPFLNWTGPPEPIAVAR
FFSASCVPGADKGQFPNLCRLCAGTGENKCAFSSQEPYFSYSGAFKCLRDGADVAFI
RESTVFEDLSDEAERDEYELLCPDNTRKPVDKFKDCHLARVPSHAVVARSVNGKED
AIWNLLRQAQEKFGKDKSPKFQLFGSPSGQKDLLFKDSAIGFSRVPPRIDSGLYLGSG
YFTAIQNLRKSEEEVAARRARVVWCAVGEQELRKCNQWSGLSEGSVTCSSASTTED
CIALVLKGEADAMSLDGGYVYTAGKCGLVPVLAENYKSQQSSDPDPNCVDRVEGY
LAVAVVRRSDTSLTWNSVKGKKSCHTAVDRTAGWNIPMGLLFNQTGSCKFDEYFS
QSCAPGSDPRSNLCALCIGDEQGENKCVPNSNERYYGYTGAFRCLAEDAGDAFVKG
VTVLQNTDGNNNEAWAKDLKLADFALLCLDGKRKPVTEARSCHLAMAPNHAVVS
RMDKVERLKQVLLHQQAKFGRNGSDCPDKFCLFQSETKNLLFNDNTECLARLHGKT
TYEKYLGPQYVAGITNLKKCSTS PLLEACEFL K- C terminal end
Yellow domain= Transferrin like domain 1H
Blue domain= Transferring like domain 2
No glycosylation or disulphide bridges have been observed in the primary structure of
lactoferrin.

3MEDICAL
Answer 2
Mature protein sequence
20 30 40 50
G RRRRSVQWCA VSQPEATKCF QWQRNMRRVR
60 70 80 90 100
GPPVSCIKRD SPIQCIQAIA ENRADAVTLD GGFIYEAGLA PYKLRPVAAE
110 120 130 140 150
VYGTERQPRT HYYAVAVVKK GGSFQLNELQ GLKSCHTGLR RNAGWNVPIG
160 170 180 190 200
TLRPFLNWTG PPEPIEAAVA RFFSASCVPG ADKGQFPNLC RLCAGTGENK
210 220 230 240 250
CAFSSQEPYF SYSGAFKCLR DGAGDVAFIR ESTVFEDLSD EAERDEYELL
260 270 280 290 300
CPDNTRKPVD KFKDCHLARV PSHAVVARSV NGKEDAIWNL LRQAQEKFGK
310 320 330 340 350
DKSPKFQLFG SPSGQKDLLF KDSAIGFSRV PPRIDSGLYL GSGYFTAIQN
360 370 380 390 400
LRKSEEEVAA RRARVVWCAV GEQELRKCNQ WSGLSEGSVT CSSASTTEDC
410 420 430 440 450
IALVLKGEAD AMSLDGGYVY TAGKCGLVPV LAENYKSQQS SDPDPNCVDR
460 470 480 490 500
PVEGYLAVAV VRRSDTSLTW NSVKGKKSCH TAVDRTAGWN IPMGLLFNQT
510 520 530 540 550
GSCKFDEYFS QSCAPGSDPR SNLCALCIGD EQGENKCVPN SNERYYGYTG
560 570 580 590 600
AFRCLAEDAG DVAFVKGVTV LQNTDGNNNE AWAKDLKLAD FALLCLDGKR
610 620 630 640 650
KPVTEARSCH LAMAPNHAVV SRMDKVERLK QVLLHQQAKF GRNGSDCPDK
660 670 680 690 700
FCLFQSETKN LLFNDNTECL ARLHGKTTYE KYLGPQYVAG ITNLKKCSTS
710
PLLEACEFLR K
Answer 2
Mature protein sequence
20 30 40 50
G RRRRSVQWCA VSQPEATKCF QWQRNMRRVR
60 70 80 90 100
GPPVSCIKRD SPIQCIQAIA ENRADAVTLD GGFIYEAGLA PYKLRPVAAE
110 120 130 140 150
VYGTERQPRT HYYAVAVVKK GGSFQLNELQ GLKSCHTGLR RNAGWNVPIG
160 170 180 190 200
TLRPFLNWTG PPEPIEAAVA RFFSASCVPG ADKGQFPNLC RLCAGTGENK
210 220 230 240 250
CAFSSQEPYF SYSGAFKCLR DGAGDVAFIR ESTVFEDLSD EAERDEYELL
260 270 280 290 300
CPDNTRKPVD KFKDCHLARV PSHAVVARSV NGKEDAIWNL LRQAQEKFGK
310 320 330 340 350
DKSPKFQLFG SPSGQKDLLF KDSAIGFSRV PPRIDSGLYL GSGYFTAIQN
360 370 380 390 400
LRKSEEEVAA RRARVVWCAV GEQELRKCNQ WSGLSEGSVT CSSASTTEDC
410 420 430 440 450
IALVLKGEAD AMSLDGGYVY TAGKCGLVPV LAENYKSQQS SDPDPNCVDR
460 470 480 490 500
PVEGYLAVAV VRRSDTSLTW NSVKGKKSCH TAVDRTAGWN IPMGLLFNQT
510 520 530 540 550
GSCKFDEYFS QSCAPGSDPR SNLCALCIGD EQGENKCVPN SNERYYGYTG
560 570 580 590 600
AFRCLAEDAG DVAFVKGVTV LQNTDGNNNE AWAKDLKLAD FALLCLDGKR
610 620 630 640 650
KPVTEARSCH LAMAPNHAVV SRMDKVERLK QVLLHQQAKF GRNGSDCPDK
660 670 680 690 700
FCLFQSETKN LLFNDNTECL ARLHGKTTYE KYLGPQYVAG ITNLKKCSTS
710
PLLEACEFLR K
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
4MEDICAL
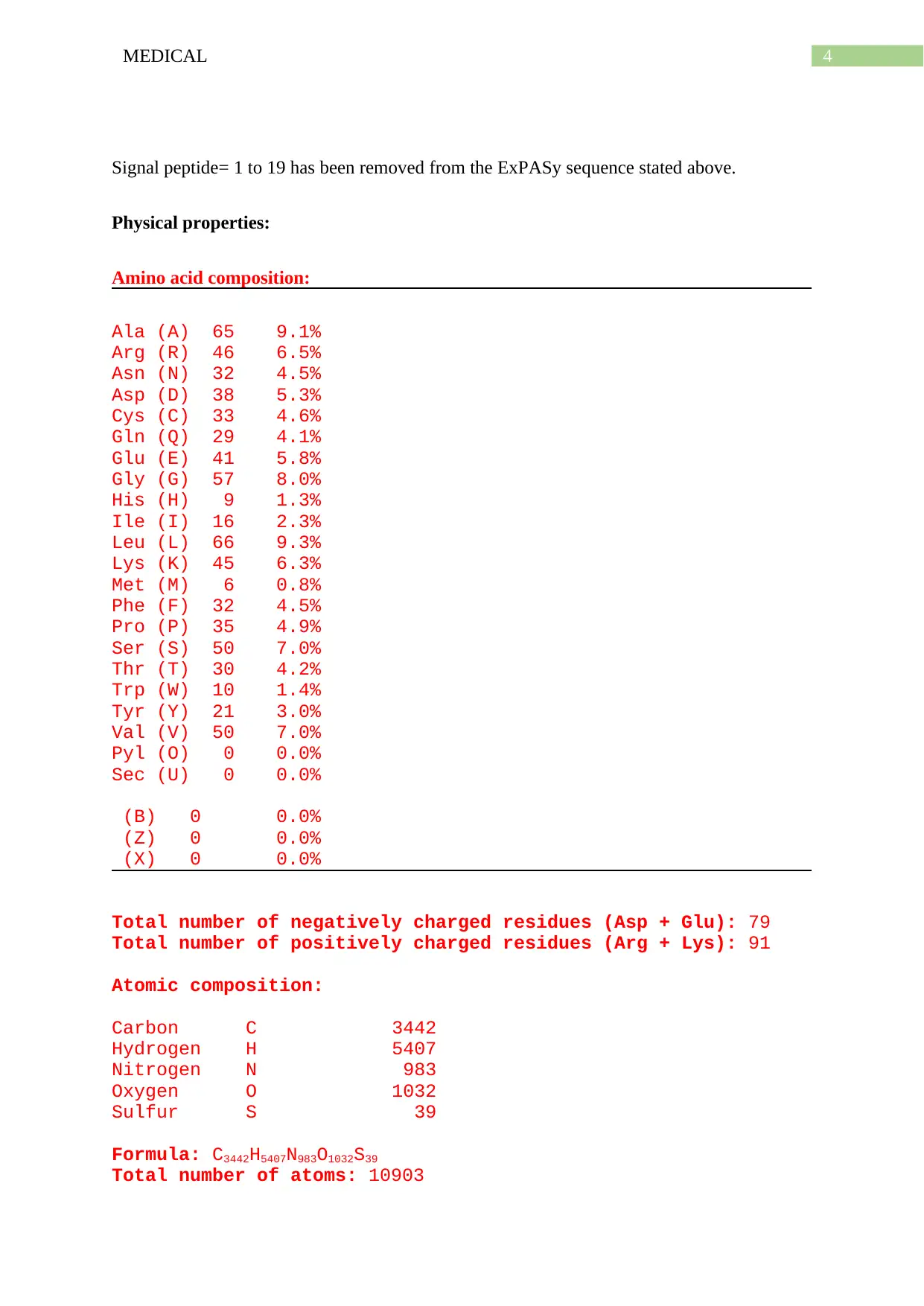
Signal peptide= 1 to 19 has been removed from the ExPASy sequence stated above.
Physical properties:
Amino acid composition:
Ala (A) 65 9.1%
Arg (R) 46 6.5%
Asn (N) 32 4.5%
Asp (D) 38 5.3%
Cys (C) 33 4.6%
Gln (Q) 29 4.1%
Glu (E) 41 5.8%
Gly (G) 57 8.0%
His (H) 9 1.3%
Ile (I) 16 2.3%
Leu (L) 66 9.3%
Lys (K) 45 6.3%
Met (M) 6 0.8%
Phe (F) 32 4.5%
Pro (P) 35 4.9%
Ser (S) 50 7.0%
Thr (T) 30 4.2%
Trp (W) 10 1.4%
Tyr (Y) 21 3.0%
Val (V) 50 7.0%
Pyl (O) 0 0.0%
Sec (U) 0 0.0%
(B) 0 0.0%
(Z) 0 0.0%
(X) 0 0.0%
Total number of negatively charged residues (Asp + Glu): 79
Total number of positively charged residues (Arg + Lys): 91
Atomic composition:
Carbon C 3442
Hydrogen H 5407
Nitrogen N 983
Oxygen O 1032
Sulfur S 39
Formula: C3442H5407N983O1032S39
Total number of atoms: 10903
Signal peptide= 1 to 19 has been removed from the ExPASy sequence stated above.
Physical properties:
Amino acid composition:
Ala (A) 65 9.1%
Arg (R) 46 6.5%
Asn (N) 32 4.5%
Asp (D) 38 5.3%
Cys (C) 33 4.6%
Gln (Q) 29 4.1%
Glu (E) 41 5.8%
Gly (G) 57 8.0%
His (H) 9 1.3%
Ile (I) 16 2.3%
Leu (L) 66 9.3%
Lys (K) 45 6.3%
Met (M) 6 0.8%
Phe (F) 32 4.5%
Pro (P) 35 4.9%
Ser (S) 50 7.0%
Thr (T) 30 4.2%
Trp (W) 10 1.4%
Tyr (Y) 21 3.0%
Val (V) 50 7.0%
Pyl (O) 0 0.0%
Sec (U) 0 0.0%
(B) 0 0.0%
(Z) 0 0.0%
(X) 0 0.0%
Total number of negatively charged residues (Asp + Glu): 79
Total number of positively charged residues (Arg + Lys): 91
Atomic composition:
Carbon C 3442
Hydrogen H 5407
Nitrogen N 983
Oxygen O 1032
Sulfur S 39
Formula: C3442H5407N983O1032S39
Total number of atoms: 10903
5MEDICAL
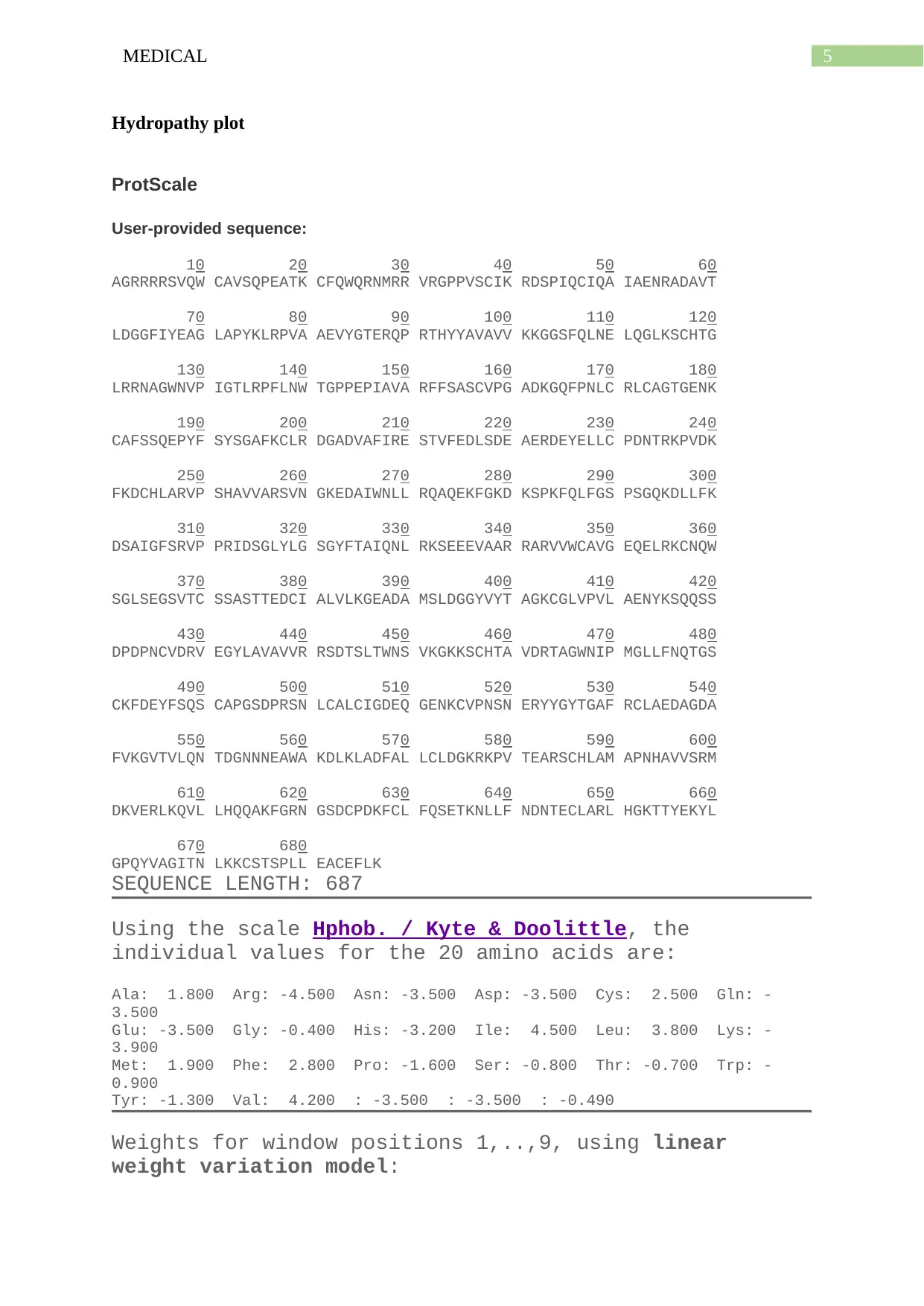
Hydropathy plot
ProtScale
User-provided sequence:
10 20 30 40 50 60
AGRRRRSVQW CAVSQPEATK CFQWQRNMRR VRGPPVSCIK RDSPIQCIQA IAENRADAVT
70 80 90 100 110 120
LDGGFIYEAG LAPYKLRPVA AEVYGTERQP RTHYYAVAVV KKGGSFQLNE LQGLKSCHTG
130 140 150 160 170 180
LRRNAGWNVP IGTLRPFLNW TGPPEPIAVA RFFSASCVPG ADKGQFPNLC RLCAGTGENK
190 200 210 220 230 240
CAFSSQEPYF SYSGAFKCLR DGADVAFIRE STVFEDLSDE AERDEYELLC PDNTRKPVDK
250 260 270 280 290 300
FKDCHLARVP SHAVVARSVN GKEDAIWNLL RQAQEKFGKD KSPKFQLFGS PSGQKDLLFK
310 320 330 340 350 360
DSAIGFSRVP PRIDSGLYLG SGYFTAIQNL RKSEEEVAAR RARVVWCAVG EQELRKCNQW
370 380 390 400 410 420
SGLSEGSVTC SSASTTEDCI ALVLKGEADA MSLDGGYVYT AGKCGLVPVL AENYKSQQSS
430 440 450 460 470 480
DPDPNCVDRV EGYLAVAVVR RSDTSLTWNS VKGKKSCHTA VDRTAGWNIP MGLLFNQTGS
490 500 510 520 530 540
CKFDEYFSQS CAPGSDPRSN LCALCIGDEQ GENKCVPNSN ERYYGYTGAF RCLAEDAGDA
550 560 570 580 590 600
FVKGVTVLQN TDGNNNEAWA KDLKLADFAL LCLDGKRKPV TEARSCHLAM APNHAVVSRM
610 620 630 640 650 660
DKVERLKQVL LHQQAKFGRN GSDCPDKFCL FQSETKNLLF NDNTECLARL HGKTTYEKYL
670 680
GPQYVAGITN LKKCSTSPLL EACEFLK
SEQUENCE LENGTH: 687
Using the scale Hphob. / Kyte & Doolittle, the
individual values for the 20 amino acids are:
Ala: 1.800 Arg: -4.500 Asn: -3.500 Asp: -3.500 Cys: 2.500 Gln: -
3.500
Glu: -3.500 Gly: -0.400 His: -3.200 Ile: 4.500 Leu: 3.800 Lys: -
3.900
Met: 1.900 Phe: 2.800 Pro: -1.600 Ser: -0.800 Thr: -0.700 Trp: -
0.900
Tyr: -1.300 Val: 4.200 : -3.500 : -3.500 : -0.490
Weights for window positions 1,..,9, using linear
weight variation model:
Hydropathy plot
ProtScale
User-provided sequence:
10 20 30 40 50 60
AGRRRRSVQW CAVSQPEATK CFQWQRNMRR VRGPPVSCIK RDSPIQCIQA IAENRADAVT
70 80 90 100 110 120
LDGGFIYEAG LAPYKLRPVA AEVYGTERQP RTHYYAVAVV KKGGSFQLNE LQGLKSCHTG
130 140 150 160 170 180
LRRNAGWNVP IGTLRPFLNW TGPPEPIAVA RFFSASCVPG ADKGQFPNLC RLCAGTGENK
190 200 210 220 230 240
CAFSSQEPYF SYSGAFKCLR DGADVAFIRE STVFEDLSDE AERDEYELLC PDNTRKPVDK
250 260 270 280 290 300
FKDCHLARVP SHAVVARSVN GKEDAIWNLL RQAQEKFGKD KSPKFQLFGS PSGQKDLLFK
310 320 330 340 350 360
DSAIGFSRVP PRIDSGLYLG SGYFTAIQNL RKSEEEVAAR RARVVWCAVG EQELRKCNQW
370 380 390 400 410 420
SGLSEGSVTC SSASTTEDCI ALVLKGEADA MSLDGGYVYT AGKCGLVPVL AENYKSQQSS
430 440 450 460 470 480
DPDPNCVDRV EGYLAVAVVR RSDTSLTWNS VKGKKSCHTA VDRTAGWNIP MGLLFNQTGS
490 500 510 520 530 540
CKFDEYFSQS CAPGSDPRSN LCALCIGDEQ GENKCVPNSN ERYYGYTGAF RCLAEDAGDA
550 560 570 580 590 600
FVKGVTVLQN TDGNNNEAWA KDLKLADFAL LCLDGKRKPV TEARSCHLAM APNHAVVSRM
610 620 630 640 650 660
DKVERLKQVL LHQQAKFGRN GSDCPDKFCL FQSETKNLLF NDNTECLARL HGKTTYEKYL
670 680
GPQYVAGITN LKKCSTSPLL EACEFLK
SEQUENCE LENGTH: 687
Using the scale Hphob. / Kyte & Doolittle, the
individual values for the 20 amino acids are:
Ala: 1.800 Arg: -4.500 Asn: -3.500 Asp: -3.500 Cys: 2.500 Gln: -
3.500
Glu: -3.500 Gly: -0.400 His: -3.200 Ile: 4.500 Leu: 3.800 Lys: -
3.900
Met: 1.900 Phe: 2.800 Pro: -1.600 Ser: -0.800 Thr: -0.700 Trp: -
0.900
Tyr: -1.300 Val: 4.200 : -3.500 : -3.500 : -0.490
Weights for window positions 1,..,9, using linear
weight variation model:
6MEDICAL
1 2 3 4 5 6 7 8 9
1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00
edge center edge
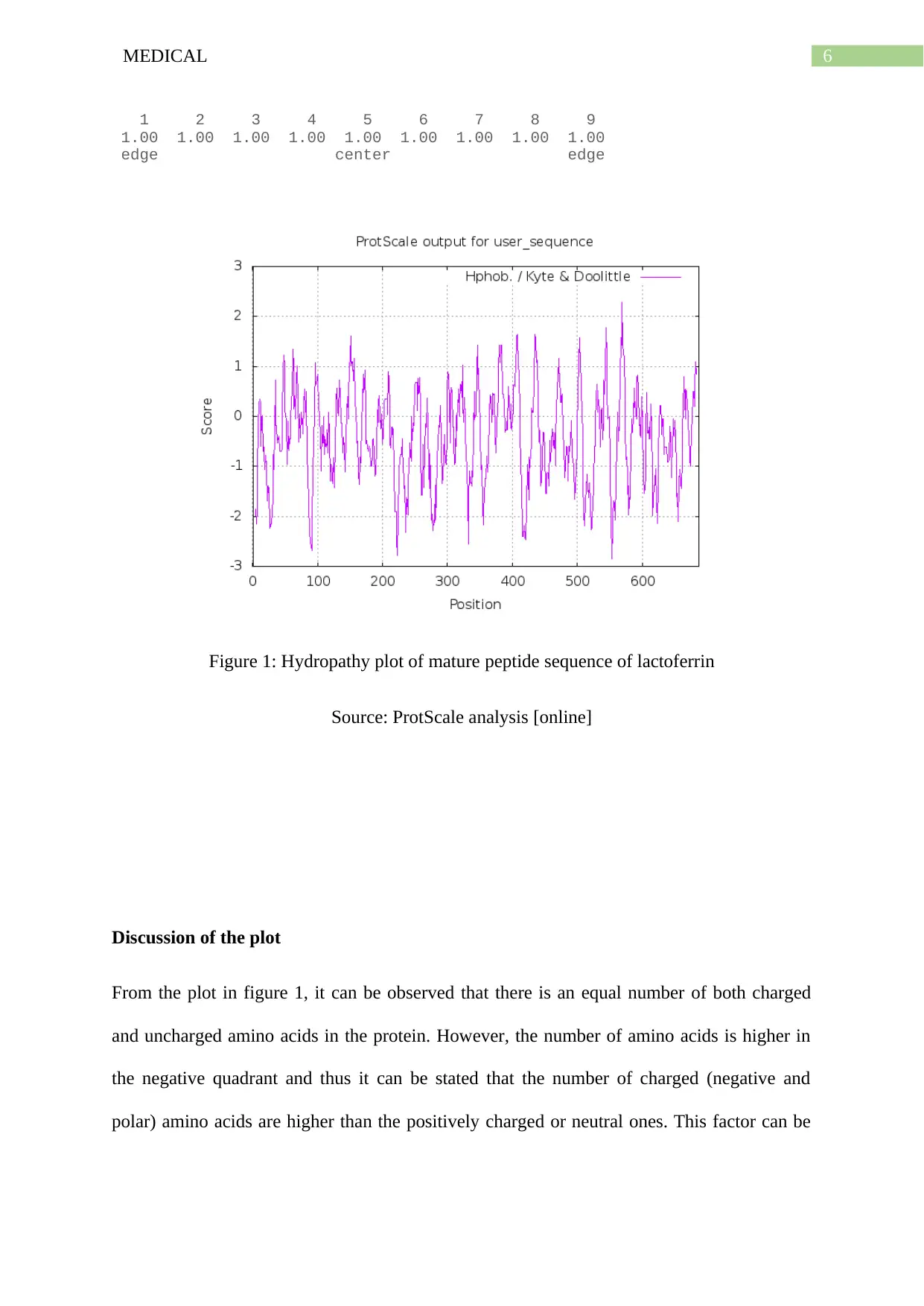
Figure 1: Hydropathy plot of mature peptide sequence of lactoferrin
Source: ProtScale analysis [online]
Discussion of the plot
From the plot in figure 1, it can be observed that there is an equal number of both charged
and uncharged amino acids in the protein. However, the number of amino acids is higher in
the negative quadrant and thus it can be stated that the number of charged (negative and
polar) amino acids are higher than the positively charged or neutral ones. This factor can be
1 2 3 4 5 6 7 8 9
1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00
edge center edge
Figure 1: Hydropathy plot of mature peptide sequence of lactoferrin
Source: ProtScale analysis [online]
Discussion of the plot
From the plot in figure 1, it can be observed that there is an equal number of both charged
and uncharged amino acids in the protein. However, the number of amino acids is higher in
the negative quadrant and thus it can be stated that the number of charged (negative and
polar) amino acids are higher than the positively charged or neutral ones. This factor can be
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7MEDICAL
stated to be justified from the separate values for amino acids in the plot, given above figure
1.
pI and molecular weight
Theoretical pI/Mw: 8.56 / 77712.42
The pI value of the mature peptide sequence states that a higher value than 7 is due to the
presence of basic amino acids. This pI value is near neutral because of the equal presence of
all the types of amino acids, neutralizing their charges.
Answer 3
Lactoferrin has been first isolated by Sorensen and Sorensen from cow milk in the year 1939.
In 1960, this protein was also spotted in human breast milk with an iron-binding affinity by
three independent laboratories. However, in the past, this protein was hard to be purified and
thus characterization was hard.
Lactoferrin containing camel milk and human colostrum was collected in
tubes.
After the adjustment, the samples are applied in a CM cellulose column
with buffer at pH= 7.8 with phosphate saline buffer. The final solution was
centrifuged and the collected supernatant was subjected to FPLC.
FPLC (Fast protein liquid chromatography was performed with FPLC
equipment. The collected elution was dissolved in storage buffer and
dialysis was run against storage buffer.
After elution of the protein components, the absorption peaks were
measured for lactoferrin and lactoperoxidase (LF and LP respectively).
After dialysis with storage buffer, a higher purity was obtained as shown
below.
Higher the purity, higher will be the peak of the absorbance curve as stated
below.
stated to be justified from the separate values for amino acids in the plot, given above figure
1.
pI and molecular weight
Theoretical pI/Mw: 8.56 / 77712.42
The pI value of the mature peptide sequence states that a higher value than 7 is due to the
presence of basic amino acids. This pI value is near neutral because of the equal presence of
all the types of amino acids, neutralizing their charges.
Answer 3
Lactoferrin has been first isolated by Sorensen and Sorensen from cow milk in the year 1939.
In 1960, this protein was also spotted in human breast milk with an iron-binding affinity by
three independent laboratories. However, in the past, this protein was hard to be purified and
thus characterization was hard.
Lactoferrin containing camel milk and human colostrum was collected in
tubes.
After the adjustment, the samples are applied in a CM cellulose column
with buffer at pH= 7.8 with phosphate saline buffer. The final solution was
centrifuged and the collected supernatant was subjected to FPLC.
FPLC (Fast protein liquid chromatography was performed with FPLC
equipment. The collected elution was dissolved in storage buffer and
dialysis was run against storage buffer.
After elution of the protein components, the absorption peaks were
measured for lactoferrin and lactoperoxidase (LF and LP respectively).
After dialysis with storage buffer, a higher purity was obtained as shown
below.
Higher the purity, higher will be the peak of the absorbance curve as stated
below.
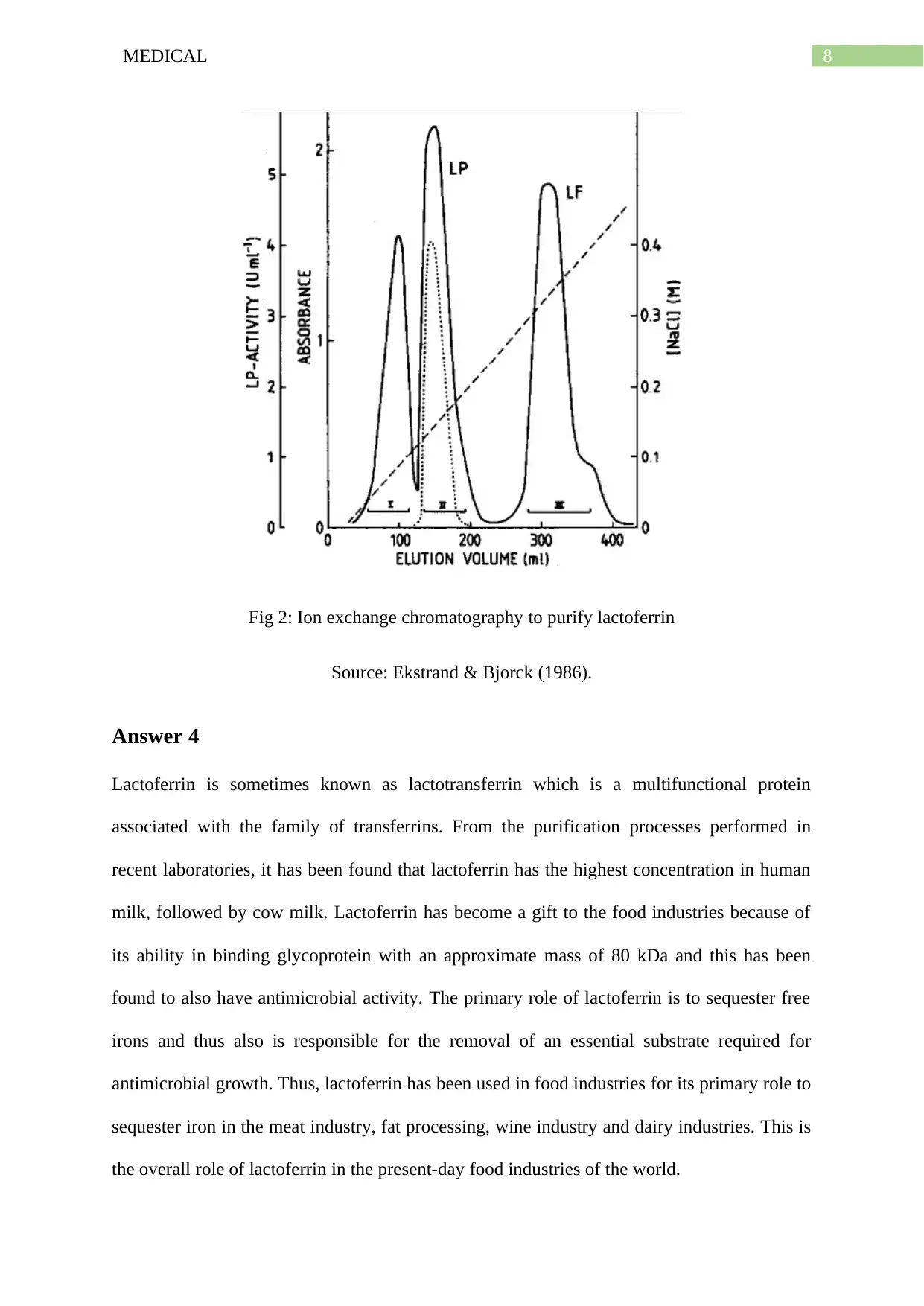
8MEDICAL
Fig 2: Ion exchange chromatography to purify lactoferrin
Source: Ekstrand & Bjorck (1986).
Answer 4
Lactoferrin is sometimes known as lactotransferrin which is a multifunctional protein
associated with the family of transferrins. From the purification processes performed in
recent laboratories, it has been found that lactoferrin has the highest concentration in human
milk, followed by cow milk. Lactoferrin has become a gift to the food industries because of
its ability in binding glycoprotein with an approximate mass of 80 kDa and this has been
found to also have antimicrobial activity. The primary role of lactoferrin is to sequester free
irons and thus also is responsible for the removal of an essential substrate required for
antimicrobial growth. Thus, lactoferrin has been used in food industries for its primary role to
sequester iron in the meat industry, fat processing, wine industry and dairy industries. This is
the overall role of lactoferrin in the present-day food industries of the world.
Fig 2: Ion exchange chromatography to purify lactoferrin
Source: Ekstrand & Bjorck (1986).
Answer 4
Lactoferrin is sometimes known as lactotransferrin which is a multifunctional protein
associated with the family of transferrins. From the purification processes performed in
recent laboratories, it has been found that lactoferrin has the highest concentration in human
milk, followed by cow milk. Lactoferrin has become a gift to the food industries because of
its ability in binding glycoprotein with an approximate mass of 80 kDa and this has been
found to also have antimicrobial activity. The primary role of lactoferrin is to sequester free
irons and thus also is responsible for the removal of an essential substrate required for
antimicrobial growth. Thus, lactoferrin has been used in food industries for its primary role to
sequester iron in the meat industry, fat processing, wine industry and dairy industries. This is
the overall role of lactoferrin in the present-day food industries of the world.
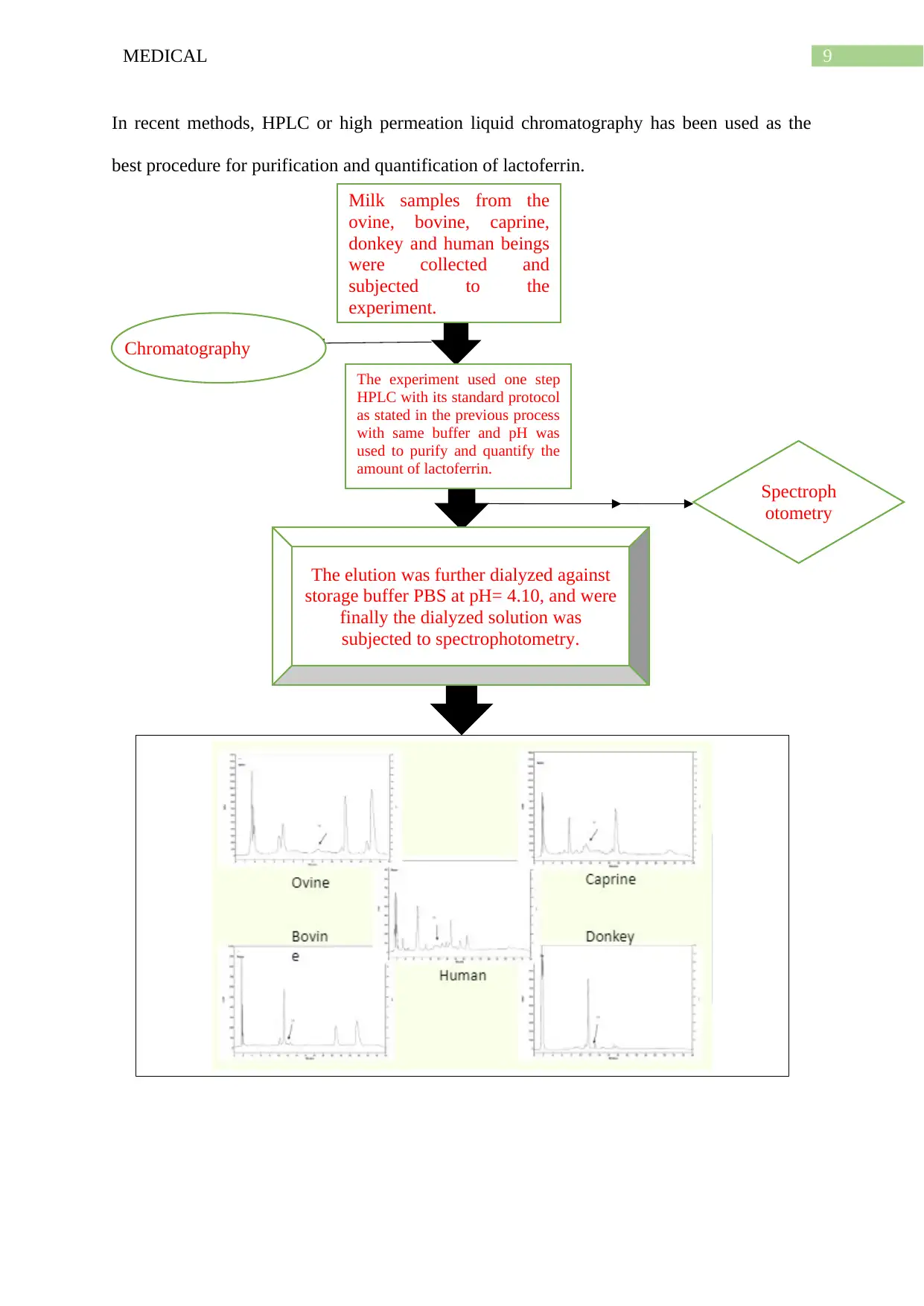
9MEDICAL
In recent methods, HPLC or high permeation liquid chromatography has been used as the
best procedure for purification and quantification of lactoferrin.
Milk samples from the
ovine, bovine, caprine,
donkey and human beings
were collected and
subjected to the
experiment.
The experiment used one step
HPLC with its standard protocol
as stated in the previous process
with same buffer and pH was
used to purify and quantify the
amount of lactoferrin.
The elution was further dialyzed against
storage buffer PBS at pH= 4.10, and were
finally the dialyzed solution was
subjected to spectrophotometry.
Chromatography
Spectroph
otometry
In recent methods, HPLC or high permeation liquid chromatography has been used as the
best procedure for purification and quantification of lactoferrin.
Milk samples from the
ovine, bovine, caprine,
donkey and human beings
were collected and
subjected to the
experiment.
The experiment used one step
HPLC with its standard protocol
as stated in the previous process
with same buffer and pH was
used to purify and quantify the
amount of lactoferrin.
The elution was further dialyzed against
storage buffer PBS at pH= 4.10, and were
finally the dialyzed solution was
subjected to spectrophotometry.
Chromatography
Spectroph
otometry
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

10MEDICAL
Bibliography
Alexander, D.B., Vogel, H.J. and Tsuda, H., 2017. Lactoferrin researchers descend on
Nagoya Castle. Biochemistry and Cell Biology, 95(1), pp.1-4.
Duran, A. and Kahve, H.I., 2017. The use of lactoferrin in food industry. Acad J Sci, 7,
pp.89-94.
Elagamy, E.I., Ruppanner, R., Ismail, A., Champagne, C.P. and Assaf, R., 1996. Purification
and characterization of lactoferrin, lactoperoxidase, lysozyme and immunoglobulins from
camel's milk. International Dairy Journal, 6(2), pp.129-145.
ExPASy
HANI, S., 2018. ASSESSMENT OF THE EFFECTS OF ACTIVATED LACTOPEROXIDASE
SYSTEM ON MICROBIOLOGICAL QUALITY OF RAW COW MILK ON DIFFERENT
CLIMATIC ZONE OF ETHIOPIA (Doctoral dissertation).
Indyk, H.E. and Filonzi, E.L., 2005. Determination of lactoferrin in bovine milk, colostrum
and infant formulas by optical biosensor analysis. International Dairy Journal, 15(5), pp.429-
438.
Kawakami, H., Shinmoto, H., Dosako, S. and Ahiko, K., Snow Brand Milk Products Co,
1987. Method for separating bovine lactoferrin from cow's milk and purifying same. U.S.
Patent 4,668,771.
Montiel, R., Martín-Cabrejas, I., Peirotén, Á. and Medina, M., 2016. Reuterin,
lactoperoxidase, lactoferrin and high hydrostatic pressure treatments on the characteristics of
cooked ham. Innovative Food Science & Emerging Technologies, 35, pp.111-118.
Bibliography
Alexander, D.B., Vogel, H.J. and Tsuda, H., 2017. Lactoferrin researchers descend on
Nagoya Castle. Biochemistry and Cell Biology, 95(1), pp.1-4.
Duran, A. and Kahve, H.I., 2017. The use of lactoferrin in food industry. Acad J Sci, 7,
pp.89-94.
Elagamy, E.I., Ruppanner, R., Ismail, A., Champagne, C.P. and Assaf, R., 1996. Purification
and characterization of lactoferrin, lactoperoxidase, lysozyme and immunoglobulins from
camel's milk. International Dairy Journal, 6(2), pp.129-145.
ExPASy
HANI, S., 2018. ASSESSMENT OF THE EFFECTS OF ACTIVATED LACTOPEROXIDASE
SYSTEM ON MICROBIOLOGICAL QUALITY OF RAW COW MILK ON DIFFERENT
CLIMATIC ZONE OF ETHIOPIA (Doctoral dissertation).
Indyk, H.E. and Filonzi, E.L., 2005. Determination of lactoferrin in bovine milk, colostrum
and infant formulas by optical biosensor analysis. International Dairy Journal, 15(5), pp.429-
438.
Kawakami, H., Shinmoto, H., Dosako, S. and Ahiko, K., Snow Brand Milk Products Co,
1987. Method for separating bovine lactoferrin from cow's milk and purifying same. U.S.
Patent 4,668,771.
Montiel, R., Martín-Cabrejas, I., Peirotén, Á. and Medina, M., 2016. Reuterin,
lactoperoxidase, lactoferrin and high hydrostatic pressure treatments on the characteristics of
cooked ham. Innovative Food Science & Emerging Technologies, 35, pp.111-118.

11MEDICAL
Tsakali, E., Stamatopoulos, K., Kitsou, A., Houhoula, D., Koulouris, S., Tsaknis, J., Van
Impe, J. and Chatzilazarou, A., 2015. Determination of Lactoferrin in milk of various species.
In Preconference workshop on Food Technology 2015 Conference, Date: 2015/06/02-
2015/06/03, Location: Athens, Greece.
Wang, G. and Guo, M., 2019. Manufacturing Technologies of Whey Protein Products. Whey
Protein Production, Chemistry, Functionality, and Applications, pp.13-37.
UniProt
Tsakali, E., Stamatopoulos, K., Kitsou, A., Houhoula, D., Koulouris, S., Tsaknis, J., Van
Impe, J. and Chatzilazarou, A., 2015. Determination of Lactoferrin in milk of various species.
In Preconference workshop on Food Technology 2015 Conference, Date: 2015/06/02-
2015/06/03, Location: Athens, Greece.
Wang, G. and Guo, M., 2019. Manufacturing Technologies of Whey Protein Products. Whey
Protein Production, Chemistry, Functionality, and Applications, pp.13-37.
UniProt
1 out of 12
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.



