SLE212 Biochemistry Worksheet B
Added on 2023-01-20
10 Pages2416 Words94 Views
SLE212 Biochemistry T1 2019 Assessment Worksheet B
Worksheet Worksheet B
Preparation of buffers and titration of amino acid
Name
Prac Partners
Prac date
Due date
1) Electronic Format
The worksheet must be produced entirely in an electronic fashion, however, we do not accept ePens! Hand-
written or hand-drawn components, be it as original or as scanned image, will not be accepted/not receive any
marks! Submit electronically as either a Word or PDF file via the designated CloudDeakin dropbox.
2) Plagiarism and Collusion
Before submitting your worksheet to the dropbox submit it to the Check Your Work: Turnitin Assignment Folder
available on CloudDeakin. In case that we find that work is the result of plagiarism (from foreign source like
internet or fellow student) or collusion we report this to the Faculty Academic Progress and Discipline Committee.Note: It is NOT sufficient to submit your worksheet only to the Turnitin Folder! Your worksheet will be marked
only if you submit it to the appropriate Dropbox!
3) Due dates for worksheet submission:
Worksheet A is due 5.00 pm two teaching weeks after practical session 1, this is the day BEFORE your prac 2
Worksheet B is due 5.00 pm two teaching weeks after practical session 2, this is the day BEFORE your prac 3
Worksheet C is due 5.00 pm two teaching weeks after practical session 4 (!), this is the day BEFORE your prac 5
Worksheet D is due 5.00 pm ONE (!) teaching week after practical session 5 (!)
4) Referencing
All foreign sources of information, i.e. information that was not provided in this unit, must
be cited. The citation style to be used is Harvard style, for more information about this style and referencing in
general see: https://www.deakin.edu.au/students/studying/study-support/referencing. Citations will be
considered only when they are placed as in-text citations such that it is clear which statement they do support.
Furthermore, bibliographic details of a citation must be listed within the question where they are used, reference
lists at the end of the worksheet will not be accepted! The citation needs to be a quality journal article, book or
refereed website. No marks are awarded if Wikipedia or poor-quality websites (eg. the website of a course at
another institution) are cited.
The practical manual, the prac script, OLMs and lecture notes can NOT be cited! Citations of text
books are often most appropriate for the level of this unit and therefore encouraged! If you do cite a
textbook you MUST indicate relevant page numbers!
5) Learning Outcomes:
Q1, 3 and 4: Quantitation skills. Application of mathematical equation and discipline specific knowledge
to quantitatively describe a system.
Q2: Written communication. Presentation of experimental data.
Q5: Critical thinking & problem solving. Use of discipline specific knowledge and logic to predict the
chemical behaviour of a biochemical compound.
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
Worksheet Worksheet B
Preparation of buffers and titration of amino acid
Name
Prac Partners
Prac date
Due date
1) Electronic Format
The worksheet must be produced entirely in an electronic fashion, however, we do not accept ePens! Hand-
written or hand-drawn components, be it as original or as scanned image, will not be accepted/not receive any
marks! Submit electronically as either a Word or PDF file via the designated CloudDeakin dropbox.
2) Plagiarism and Collusion
Before submitting your worksheet to the dropbox submit it to the Check Your Work: Turnitin Assignment Folder
available on CloudDeakin. In case that we find that work is the result of plagiarism (from foreign source like
internet or fellow student) or collusion we report this to the Faculty Academic Progress and Discipline Committee.Note: It is NOT sufficient to submit your worksheet only to the Turnitin Folder! Your worksheet will be marked
only if you submit it to the appropriate Dropbox!
3) Due dates for worksheet submission:
Worksheet A is due 5.00 pm two teaching weeks after practical session 1, this is the day BEFORE your prac 2
Worksheet B is due 5.00 pm two teaching weeks after practical session 2, this is the day BEFORE your prac 3
Worksheet C is due 5.00 pm two teaching weeks after practical session 4 (!), this is the day BEFORE your prac 5
Worksheet D is due 5.00 pm ONE (!) teaching week after practical session 5 (!)
4) Referencing
All foreign sources of information, i.e. information that was not provided in this unit, must
be cited. The citation style to be used is Harvard style, for more information about this style and referencing in
general see: https://www.deakin.edu.au/students/studying/study-support/referencing. Citations will be
considered only when they are placed as in-text citations such that it is clear which statement they do support.
Furthermore, bibliographic details of a citation must be listed within the question where they are used, reference
lists at the end of the worksheet will not be accepted! The citation needs to be a quality journal article, book or
refereed website. No marks are awarded if Wikipedia or poor-quality websites (eg. the website of a course at
another institution) are cited.
The practical manual, the prac script, OLMs and lecture notes can NOT be cited! Citations of text
books are often most appropriate for the level of this unit and therefore encouraged! If you do cite a
textbook you MUST indicate relevant page numbers!
5) Learning Outcomes:
Q1, 3 and 4: Quantitation skills. Application of mathematical equation and discipline specific knowledge
to quantitatively describe a system.
Q2: Written communication. Presentation of experimental data.
Q5: Critical thinking & problem solving. Use of discipline specific knowledge and logic to predict the
chemical behaviour of a biochemical compound.
DO NOT DELETE ANY TEXT FROM THE QUESTIONS

SLE212 Biochemistry T1 2019 Assessment Worksheet B
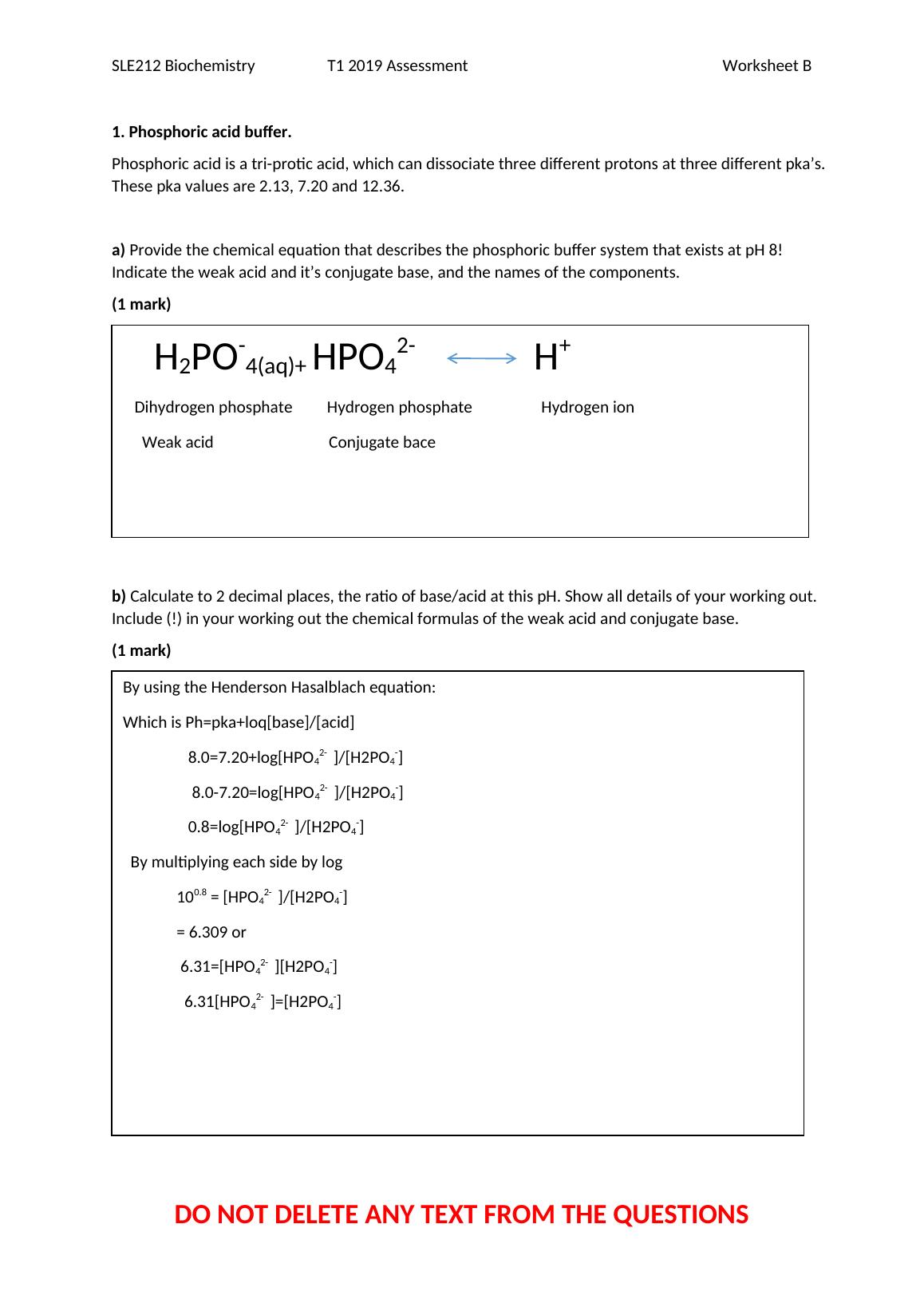
1. Phosphoric acid buffer.
Phosphoric acid is a tri-protic acid, which can dissociate three different protons at three different pka’s.
These pka values are 2.13, 7.20 and 12.36.
a) Provide the chemical equation that describes the phosphoric buffer system that exists at pH 8!
Indicate the weak acid and it’s conjugate base, and the names of the components.
(1 mark)
b) Calculate to 2 decimal places, the ratio of base/acid at this pH. Show all details of your working out.
Include (!) in your working out the chemical formulas of the weak acid and conjugate base.
(1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
H2PO-4(aq)+ HPO42- H+
Dihydrogen phosphate Hydrogen phosphate Hydrogen ion
Weak acid Conjugate bace
By using the Henderson Hasalblach equation:
Which is Ph=pka+loq[base]/[acid]
8.0=7.20+log[HPO42- ]/[H2PO4-]
8.0-7.20=log[HPO42- ]/[H2PO4-]
0.8=log[HPO42- ]/[H2PO4-]
By multiplying each side by log
100.8 = [HPO42- ]/[H2PO4-]
= 6.309 or
6.31=[HPO42- ][H2PO4-]
6.31[HPO42- ]=[H2PO4-]
1. Phosphoric acid buffer.
Phosphoric acid is a tri-protic acid, which can dissociate three different protons at three different pka’s.
These pka values are 2.13, 7.20 and 12.36.
a) Provide the chemical equation that describes the phosphoric buffer system that exists at pH 8!
Indicate the weak acid and it’s conjugate base, and the names of the components.
(1 mark)
b) Calculate to 2 decimal places, the ratio of base/acid at this pH. Show all details of your working out.
Include (!) in your working out the chemical formulas of the weak acid and conjugate base.
(1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
H2PO-4(aq)+ HPO42- H+
Dihydrogen phosphate Hydrogen phosphate Hydrogen ion
Weak acid Conjugate bace
By using the Henderson Hasalblach equation:
Which is Ph=pka+loq[base]/[acid]
8.0=7.20+log[HPO42- ]/[H2PO4-]
8.0-7.20=log[HPO42- ]/[H2PO4-]
0.8=log[HPO42- ]/[H2PO4-]
By multiplying each side by log
100.8 = [HPO42- ]/[H2PO4-]
= 6.309 or
6.31=[HPO42- ][H2PO4-]
6.31[HPO42- ]=[H2PO4-]
SLE212 Biochemistry T1 2019 Assessment Worksheet B
1c. Calculate to 2 decimal places the concentration of the conjugate acid and conjugate base for a total
buffer concentration of 200 mM. Show all details of your working out, including (!) the chemical
formula of the acid and base, include all units and state the answer in a complete sentence.
(1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
Buffer concentration =[H2PO4-]+[HPO32-]= 200mM bu converting it =0.2M
1) From equation2:
[H2PO4-]+[HPO32-]=0.2M
2)From equation1(from part b)
6.31[H2PO4-]=[HPO32-]
- By combining (1)and (2) :
[H2PO4-]+6.31[H2PO4-]=0.2 M
7.31 [H2PO4-]=0.2 M
[H2PO4-]=(0.2/7.31M
Gives [H2PO4-]=0.03M
- using the concentration of acid into equation 2 gives :
0.03M +[ HPO32-]=0.2 M
[ HPO32-]=0.2-0.03M
[ HPO32-]=0.17 M
This means that the consentration on a conjugated acid and the conjugated base in this buffer
is 0.03 M and 0.17 M respectively.
1c. Calculate to 2 decimal places the concentration of the conjugate acid and conjugate base for a total
buffer concentration of 200 mM. Show all details of your working out, including (!) the chemical
formula of the acid and base, include all units and state the answer in a complete sentence.
(1 mark)
DO NOT DELETE ANY TEXT FROM THE QUESTIONS
Buffer concentration =[H2PO4-]+[HPO32-]= 200mM bu converting it =0.2M
1) From equation2:
[H2PO4-]+[HPO32-]=0.2M
2)From equation1(from part b)
6.31[H2PO4-]=[HPO32-]
- By combining (1)and (2) :
[H2PO4-]+6.31[H2PO4-]=0.2 M
7.31 [H2PO4-]=0.2 M
[H2PO4-]=(0.2/7.31M
Gives [H2PO4-]=0.03M
- using the concentration of acid into equation 2 gives :
0.03M +[ HPO32-]=0.2 M
[ HPO32-]=0.2-0.03M
[ HPO32-]=0.17 M
This means that the consentration on a conjugated acid and the conjugated base in this buffer
is 0.03 M and 0.17 M respectively.

End of preview
Want to access all the pages? Upload your documents or become a member.
Related Documents
Biochemical Fractionation of Milk Proteinslg...
|10
|2482
|38
Assignment About Biochemistrylg...
|11
|3404
|15