Enthalpy of Combustion in Different Alcohol Chains and Branches
VerifiedAdded on 2023/04/22
|14
|3827
|499
AI Summary
This article explores the relationship between the number of carbon atoms in an alcohol and the energy released as a result of enthalpy change during combustion. It discusses the molecular formulae for different alcohols, the combustion reaction for alcohols, and the impact of the number of carbon atoms on enthalpy change. The article also includes a methodology section and data analysis.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

CHANGES IN ENTHALPY OF COMBUSTION IN DIFFERENT ALCOHOL CHAIN AND
BRANCHES AS THE CARBON ATOMS AND BRANCH CONFIGURATION CHANGES
Introduction
Alcohols refer to a group of organic compounds known as hydrocarbons that contain
the –OH group. The group comprises of three major elements, namely Carbon, Oxygen, and
Hydrogen while the -OH group contributes by determining the manner in which the
hydrocarbon compound reacts. This group has a general chemical formula of CnH2n+1OH
where the value of n represents the number of carbon atoms in the compound. Since
alcohols are organic compounds, their combustion is known to produce high amounts of
energy because their combustion reactions are exothermic (Bruno and Smith, 2016, p.
2109). An exothermic chemical is that which releases energy when it is broken down in the
presence of oxygen as in the combustion reaction. This exothermic trait of fuels does not
guarantee that the alcohols will be fuels that will release the highest amount of energy
which makes the best fuels. The amount of energy released in an exothermic reaction is
determined by the enthalpy change that occurs when the compound is combusted.
Enthalpy is a term used to define the amount of heat energy that will be yielded when one
mole of a substance is combusted in the presence of oxygen (Wiberg, Crocker, and Morgan,
2016, p. 2147).
Since enthalpy change is affected by the mole ratios of the reactants in this combustion
reaction, the molecular mass of the compound has an impact on the values of enthalpy that
are attained when a compound is combusted (Bruno and Smith, 2016, p. 2111). This is
because the alcohol chain to be combusted continues to become longer as a unit
CH2(methylene) molecule continues to be added to the chain. This implies that much more
energy will be required to break these long chains and more covalent bonds and Van der
Waal’s forces in the compound (Thornton, 2017, p. 197). As the number of carbon atoms in
the alcohols continues to increase, the enthalpy of the oxidation reaction continues to
decrease thus making the enthalpy of combustion to become more negative increasing
carbon atoms in the chain. The impact of an even higher enthalpy change with every
increase in the carbon atom in the long hydrocarbon chains is a longer time taken for the
water to reach the boiling point (Thornton, 2017, p. 199). This will indicate that the fuel is
BRANCHES AS THE CARBON ATOMS AND BRANCH CONFIGURATION CHANGES
Introduction
Alcohols refer to a group of organic compounds known as hydrocarbons that contain
the –OH group. The group comprises of three major elements, namely Carbon, Oxygen, and
Hydrogen while the -OH group contributes by determining the manner in which the
hydrocarbon compound reacts. This group has a general chemical formula of CnH2n+1OH
where the value of n represents the number of carbon atoms in the compound. Since
alcohols are organic compounds, their combustion is known to produce high amounts of
energy because their combustion reactions are exothermic (Bruno and Smith, 2016, p.
2109). An exothermic chemical is that which releases energy when it is broken down in the
presence of oxygen as in the combustion reaction. This exothermic trait of fuels does not
guarantee that the alcohols will be fuels that will release the highest amount of energy
which makes the best fuels. The amount of energy released in an exothermic reaction is
determined by the enthalpy change that occurs when the compound is combusted.
Enthalpy is a term used to define the amount of heat energy that will be yielded when one
mole of a substance is combusted in the presence of oxygen (Wiberg, Crocker, and Morgan,
2016, p. 2147).
Since enthalpy change is affected by the mole ratios of the reactants in this combustion
reaction, the molecular mass of the compound has an impact on the values of enthalpy that
are attained when a compound is combusted (Bruno and Smith, 2016, p. 2111). This is
because the alcohol chain to be combusted continues to become longer as a unit
CH2(methylene) molecule continues to be added to the chain. This implies that much more
energy will be required to break these long chains and more covalent bonds and Van der
Waal’s forces in the compound (Thornton, 2017, p. 197). As the number of carbon atoms in
the alcohols continues to increase, the enthalpy of the oxidation reaction continues to
decrease thus making the enthalpy of combustion to become more negative increasing
carbon atoms in the chain. The impact of an even higher enthalpy change with every
increase in the carbon atom in the long hydrocarbon chains is a longer time taken for the
water to reach the boiling point (Thornton, 2017, p. 199). This will indicate that the fuel is
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
taking even longer to break the compound bonds during the exothermic combustion
reaction which releases the heat required to boil the water in the calorimeter.
Hypothesis
The enthalpy of combustion increases as the number of carbon atoms in the alcohol chains
continues to increase and as the molecular structure of the alcohol changes to increase the
number of hydrogen bonds.
Theory
The molecular formulae for the alcohols that will be considered are as follows. These
formulae are important for the computation of the molecular masses of the alcohols.
Alcohol Chemical Formula Molecular Mass
Methanol CH3OH 32
Ethanol CH3 CH2OH 44
Propan-1-ol CH3 CH2 CH2OH 60
Propan-2-ol (CH3)2CHOH 60
Butan-1-ol CH3(CH2)3OH 74
Pentan-1-ol CH3(CH2)4OH 88
Hexan-1-ol CH3(CH2)5OH 102
The above table is able to demonstrates that as the carbon atoms increase in this
homologous group, the chains become complex and longer, and this in effect changes the
physical properties of the alcohols as you go down the homologous group.
Combustion can be defined as the chemical reaction that occurs when carbon compounds
are oxidized in the presence of oxygen to form CO2 and H2O. The combustion reaction for
alcohols follows the following chemical formula.
CnH2n+1OH n CO2 + 2n H2O
Since the reaction is exothermic, heat energy is also yielded in this process. Enthalpy, on the
other hand, refers to the amount of heat energy that will be yielded when one mole of a
substance is combusted in the presence of oxygen (Wiberg, Crocker, and Morgan, 2016, p.
2147). Since as the carbon atoms increase, the amount covalent and hydrogen bonds as
Oxygen
reaction which releases the heat required to boil the water in the calorimeter.
Hypothesis
The enthalpy of combustion increases as the number of carbon atoms in the alcohol chains
continues to increase and as the molecular structure of the alcohol changes to increase the
number of hydrogen bonds.
Theory
The molecular formulae for the alcohols that will be considered are as follows. These
formulae are important for the computation of the molecular masses of the alcohols.
Alcohol Chemical Formula Molecular Mass
Methanol CH3OH 32
Ethanol CH3 CH2OH 44
Propan-1-ol CH3 CH2 CH2OH 60
Propan-2-ol (CH3)2CHOH 60
Butan-1-ol CH3(CH2)3OH 74
Pentan-1-ol CH3(CH2)4OH 88
Hexan-1-ol CH3(CH2)5OH 102
The above table is able to demonstrates that as the carbon atoms increase in this
homologous group, the chains become complex and longer, and this in effect changes the
physical properties of the alcohols as you go down the homologous group.
Combustion can be defined as the chemical reaction that occurs when carbon compounds
are oxidized in the presence of oxygen to form CO2 and H2O. The combustion reaction for
alcohols follows the following chemical formula.
CnH2n+1OH n CO2 + 2n H2O
Since the reaction is exothermic, heat energy is also yielded in this process. Enthalpy, on the
other hand, refers to the amount of heat energy that will be yielded when one mole of a
substance is combusted in the presence of oxygen (Wiberg, Crocker, and Morgan, 2016, p.
2147). Since as the carbon atoms increase, the amount covalent and hydrogen bonds as
Oxygen

well as the Van der Waals forces to be broken down during the combustion reaction also
increase. This implies that less energy will be released per mole of alcohol being burnt as
you go down the homologous group and thus the enthalpy change will thus become more
and more negative with the increasing number of carbon atoms in the chain.
Research Question
Is there a possible relationship between the number of carbon atoms in an alcohol and the
energy released as a result of enthalpy change during combustion?
Variables
Independent Variable--The independent variables are the different types of alcohols whose
enthalpy will be tested. They are: Methanol, Ethanol, Propan-1-ol, propan-2-ol-butan-1-ol,
Pentan-1-ol and Hexan-1-ol
Dependent Variable-- The dependent variable will be the amount of the alcohol that was
burnt during the combustion reaction
Controlled Variables--These include the experiment parameters that will not be altered
during the experiment. They include the temperature increase in the water, the distance
between the bottom of the copper calorimeter and the burner, the mass of the water, the
length of time it took for the water in the calorimeter to come to a boil,the mass of the
alcohol to be burnt, the apparatus that will be utilized for the experiment, the
environmental conditions of temperature and pressure during the experiment.
Safety
The alcohol which serves as the independent variable in this experiment is changed through
replacing the alcohol being used with the next specimen with a different amount of carbon
atom. The safety of the experiment will also further be reinforced by ensuring that the
temperature increase is recorded with a thermometer and then the change in mass is
recorded on a balance away from the source of heat. To ensure that there are minimal
changes in the recorded data, the balance, burner, thermometer, and even the calorimeter
used ought to be the same one for each trial. Changes in the temperatures recorded will
also be maintained through encouraging air conditioning in the environment and the use of
increase. This implies that less energy will be released per mole of alcohol being burnt as
you go down the homologous group and thus the enthalpy change will thus become more
and more negative with the increasing number of carbon atoms in the chain.
Research Question
Is there a possible relationship between the number of carbon atoms in an alcohol and the
energy released as a result of enthalpy change during combustion?
Variables
Independent Variable--The independent variables are the different types of alcohols whose
enthalpy will be tested. They are: Methanol, Ethanol, Propan-1-ol, propan-2-ol-butan-1-ol,
Pentan-1-ol and Hexan-1-ol
Dependent Variable-- The dependent variable will be the amount of the alcohol that was
burnt during the combustion reaction
Controlled Variables--These include the experiment parameters that will not be altered
during the experiment. They include the temperature increase in the water, the distance
between the bottom of the copper calorimeter and the burner, the mass of the water, the
length of time it took for the water in the calorimeter to come to a boil,the mass of the
alcohol to be burnt, the apparatus that will be utilized for the experiment, the
environmental conditions of temperature and pressure during the experiment.
Safety
The alcohol which serves as the independent variable in this experiment is changed through
replacing the alcohol being used with the next specimen with a different amount of carbon
atom. The safety of the experiment will also further be reinforced by ensuring that the
temperature increase is recorded with a thermometer and then the change in mass is
recorded on a balance away from the source of heat. To ensure that there are minimal
changes in the recorded data, the balance, burner, thermometer, and even the calorimeter
used ought to be the same one for each trial. Changes in the temperatures recorded will
also be maintained through encouraging air conditioning in the environment and the use of

a ruler to ensure the distance between the burner and the calorimeter (Anderson, 2016, p.
4807). This distance ought to be kept as constant as possible since when it varies, the
amount of heat lost to the environment and the amount of heat energy heating the water in
the calorimeter also vary. This variation may impact the results of the experiment by causing
the temperature rise in the experiment to be inaccurately observed and thus cause the final
computations of the enthalpy to be inaccurate.
For the sake of personal safety, I wore protective equipment’s like lab coats, and goggles in
the lab as well as an emphasis placed on the use tongs to handle the hot apparatus were
emphasized to avoid any risk of injury. This is because some alcohols have the ability to
corrode the skin of its users to ensure that safety in the lab is guaranteed and optimized.
Materials
Weighing Balance (±0.001g)
Thermometer (±0.05 °C)
Copper calorimeter
Spirit Lamp
Tongs
Clamp and stand
50 ml Methanol
50 ml Ethanol
50 ml Propan-1-ol
50 ml Propan-2-ol
50 ml Butan-1-ol
50 ml pentan-1-ol
50 ml Hexan-1-ol
Match sticks
1l Distilled water
Methodology
I measured 50 cm3 of methan-1-ol and poured it into the spirit lamp burner. I then weighed
the on the weighing balance and recorded the new weight. 100g of water was then
measured on an electronic weighing balance and poured into the copper calorimeter that I
had earlier clamped to a waiting stand. It was necessary that I covered the alcohols in this
stage as they might have evaporated due to their volatile nature. The initial temperature of
the water in the calorimeter was taken and recorded. I then went ahead to insulate the
calorimeter and covered it then placed the thermometer and stirrer inside it. The burner
was then lit and the calorimeter containing the water placed over it. I immediately began
timing with a stopwatch when the alcohol was lit up. I followed to observe the temperature
4807). This distance ought to be kept as constant as possible since when it varies, the
amount of heat lost to the environment and the amount of heat energy heating the water in
the calorimeter also vary. This variation may impact the results of the experiment by causing
the temperature rise in the experiment to be inaccurately observed and thus cause the final
computations of the enthalpy to be inaccurate.
For the sake of personal safety, I wore protective equipment’s like lab coats, and goggles in
the lab as well as an emphasis placed on the use tongs to handle the hot apparatus were
emphasized to avoid any risk of injury. This is because some alcohols have the ability to
corrode the skin of its users to ensure that safety in the lab is guaranteed and optimized.
Materials
Weighing Balance (±0.001g)
Thermometer (±0.05 °C)
Copper calorimeter
Spirit Lamp
Tongs
Clamp and stand
50 ml Methanol
50 ml Ethanol
50 ml Propan-1-ol
50 ml Propan-2-ol
50 ml Butan-1-ol
50 ml pentan-1-ol
50 ml Hexan-1-ol
Match sticks
1l Distilled water
Methodology
I measured 50 cm3 of methan-1-ol and poured it into the spirit lamp burner. I then weighed
the on the weighing balance and recorded the new weight. 100g of water was then
measured on an electronic weighing balance and poured into the copper calorimeter that I
had earlier clamped to a waiting stand. It was necessary that I covered the alcohols in this
stage as they might have evaporated due to their volatile nature. The initial temperature of
the water in the calorimeter was taken and recorded. I then went ahead to insulate the
calorimeter and covered it then placed the thermometer and stirrer inside it. The burner
was then lit and the calorimeter containing the water placed over it. I immediately began
timing with a stopwatch when the alcohol was lit up. I followed to observe the temperature
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
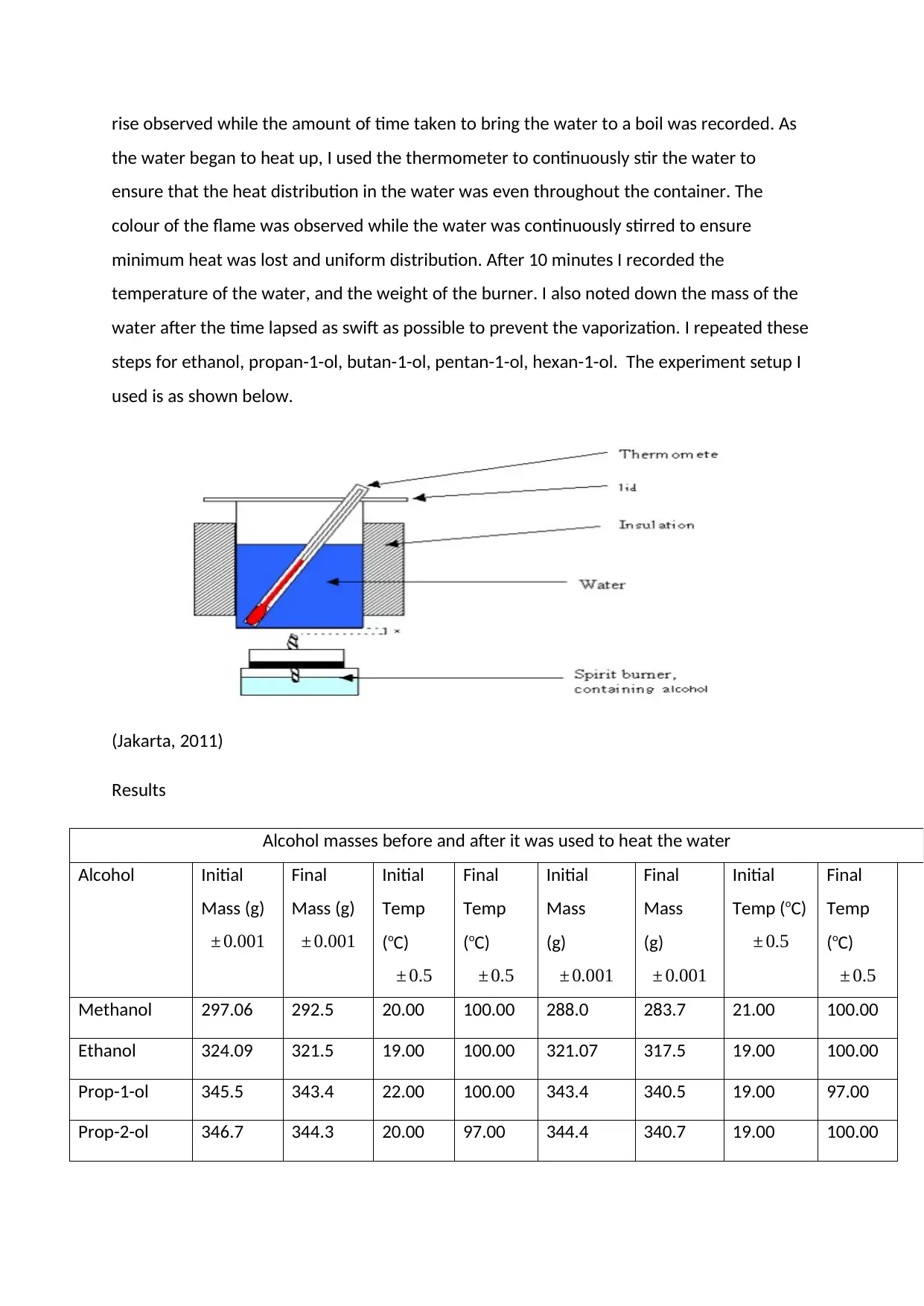
rise observed while the amount of time taken to bring the water to a boil was recorded. As
the water began to heat up, I used the thermometer to continuously stir the water to
ensure that the heat distribution in the water was even throughout the container. The
colour of the flame was observed while the water was continuously stirred to ensure
minimum heat was lost and uniform distribution. After 10 minutes I recorded the
temperature of the water, and the weight of the burner. I also noted down the mass of the
water after the time lapsed as swift as possible to prevent the vaporization. I repeated these
steps for ethanol, propan-1-ol, butan-1-ol, pentan-1-ol, hexan-1-ol. The experiment setup I
used is as shown below.
(Jakarta, 2011)
Results
Alcohol masses before and after it was used to heat the water
Alcohol Initial
Mass (g)
± 0.001
Final
Mass (g)
± 0.001
Initial
Temp
(oC)
± 0.5
Final
Temp
(oC)
± 0.5
Initial
Mass
(g)
± 0.001
Final
Mass
(g)
± 0.001
Initial
Temp (oC)
± 0.5
Final
Temp
(oC)
± 0.5
Methanol 297.06 292.5 20.00 100.00 288.0 283.7 21.00 100.00
Ethanol 324.09 321.5 19.00 100.00 321.07 317.5 19.00 100.00
Prop-1-ol 345.5 343.4 22.00 100.00 343.4 340.5 19.00 97.00
Prop-2-ol 346.7 344.3 20.00 97.00 344.4 340.7 19.00 100.00
the water began to heat up, I used the thermometer to continuously stir the water to
ensure that the heat distribution in the water was even throughout the container. The
colour of the flame was observed while the water was continuously stirred to ensure
minimum heat was lost and uniform distribution. After 10 minutes I recorded the
temperature of the water, and the weight of the burner. I also noted down the mass of the
water after the time lapsed as swift as possible to prevent the vaporization. I repeated these
steps for ethanol, propan-1-ol, butan-1-ol, pentan-1-ol, hexan-1-ol. The experiment setup I
used is as shown below.
(Jakarta, 2011)
Results
Alcohol masses before and after it was used to heat the water
Alcohol Initial
Mass (g)
± 0.001
Final
Mass (g)
± 0.001
Initial
Temp
(oC)
± 0.5
Final
Temp
(oC)
± 0.5
Initial
Mass
(g)
± 0.001
Final
Mass
(g)
± 0.001
Initial
Temp (oC)
± 0.5
Final
Temp
(oC)
± 0.5
Methanol 297.06 292.5 20.00 100.00 288.0 283.7 21.00 100.00
Ethanol 324.09 321.5 19.00 100.00 321.07 317.5 19.00 100.00
Prop-1-ol 345.5 343.4 22.00 100.00 343.4 340.5 19.00 97.00
Prop-2-ol 346.7 344.3 20.00 97.00 344.4 340.7 19.00 100.00
Butan-1-ol 345.006 342.8 21.00 99.00 342.7 339.6 20.00 98.00
Pentan-1-ol 337.9 337.0 20.00 78.00 337.0 334.1 21.00 70.00
Hexan-1-ol 346.0 345.4 20.00 75.00 335.01 334.9 20.00 71.00
Qualitative Observations
Some of the qualitative observations that I observed in this experiment include:
the inability to achieve complete combustion of the alcohols whose combustibility is
being analysed.
This was characterized by the occurrence of a yellow flame
The flame also caused soot to be formed in this process as the fuel was not able to
get enough oxygen to burn down completely combust the fuel and use it efficiently
and release the largest amount of energy.
Data Analysis
Mass Diff
(g)
± 0.001
Temp Diff
(oC)
± 0.5
Mass Diff
(g)
± 0.001
Temp Diff
(oC)
± 0.5
Mass Diff
Mean (g)
± 0.001
Temp Diff
mean
(oC)
± 0.5
Methanol 4.605 80.00 4.255 79.00 4.4300 79.50
Ethanol 2.621 81.00 3.604 81.00 3.1125 81.00
Prop-1-ol 2.089 78.00 2.851 78.00 2.470 78.00
Prop-2-ol 2.401 77.00 3.678 81.00 3.0395 79.00
Butan-1-ol 2.215 78.00 3.16 78.00 2.6875 78.00
Pentan-1-ol 0.962 58.00 2.819 49.00 1.8905 53.50
Hexan-1-ol 0.560 55.00 0.146 51.00 0.3530 53.00
To compute the uncertainties:
For mass
Pentan-1-ol 337.9 337.0 20.00 78.00 337.0 334.1 21.00 70.00
Hexan-1-ol 346.0 345.4 20.00 75.00 335.01 334.9 20.00 71.00
Qualitative Observations
Some of the qualitative observations that I observed in this experiment include:
the inability to achieve complete combustion of the alcohols whose combustibility is
being analysed.
This was characterized by the occurrence of a yellow flame
The flame also caused soot to be formed in this process as the fuel was not able to
get enough oxygen to burn down completely combust the fuel and use it efficiently
and release the largest amount of energy.
Data Analysis
Mass Diff
(g)
± 0.001
Temp Diff
(oC)
± 0.5
Mass Diff
(g)
± 0.001
Temp Diff
(oC)
± 0.5
Mass Diff
Mean (g)
± 0.001
Temp Diff
mean
(oC)
± 0.5
Methanol 4.605 80.00 4.255 79.00 4.4300 79.50
Ethanol 2.621 81.00 3.604 81.00 3.1125 81.00
Prop-1-ol 2.089 78.00 2.851 78.00 2.470 78.00
Prop-2-ol 2.401 77.00 3.678 81.00 3.0395 79.00
Butan-1-ol 2.215 78.00 3.16 78.00 2.6875 78.00
Pentan-1-ol 0.962 58.00 2.819 49.00 1.8905 53.50
Hexan-1-ol 0.560 55.00 0.146 51.00 0.3530 53.00
To compute the uncertainties:
For mass
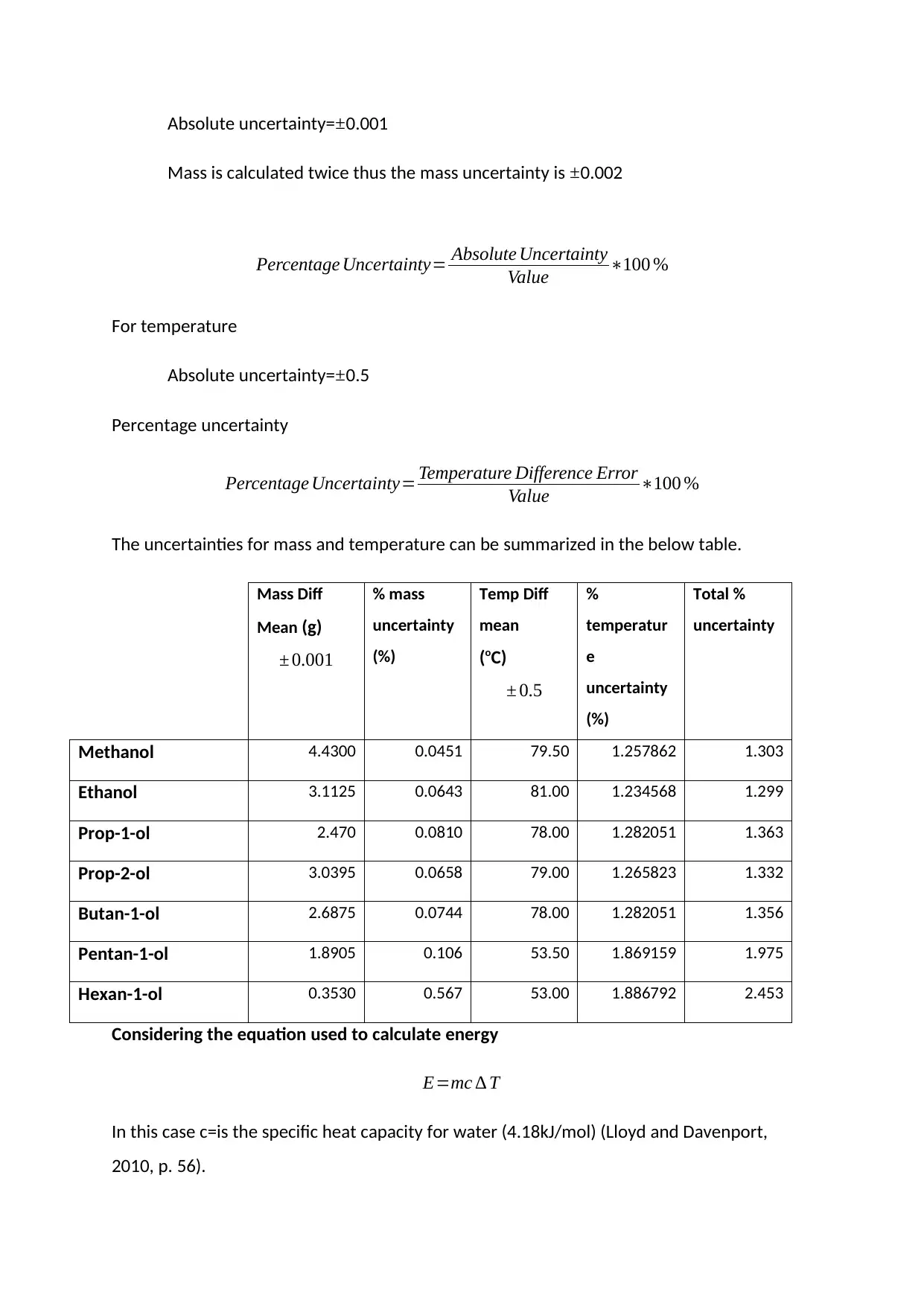
Absolute uncertainty=±0.001
Mass is calculated twice thus the mass uncertainty is ±0.002
Percentage Uncertainty= Absolute Uncertainty
Value ∗100 %
For temperature
Absolute uncertainty= ±0.5
Percentage uncertainty
Percentage Uncertainty= Temperature Difference Error
Value ∗100 %
The uncertainties for mass and temperature can be summarized in the below table.
Mass Diff
Mean (g)
± 0.001
% mass
uncertainty
(%)
Temp Diff
mean
(oC)
± 0.5
%
temperatur
e
uncertainty
(%)
Total %
uncertainty
Methanol 4.4300 0.0451 79.50 1.257862 1.303
Ethanol 3.1125 0.0643 81.00 1.234568 1.299
Prop-1-ol 2.470 0.0810 78.00 1.282051 1.363
Prop-2-ol 3.0395 0.0658 79.00 1.265823 1.332
Butan-1-ol 2.6875 0.0744 78.00 1.282051 1.356
Pentan-1-ol 1.8905 0.106 53.50 1.869159 1.975
Hexan-1-ol 0.3530 0.567 53.00 1.886792 2.453
Considering the equation used to calculate energy
E=mc ∆ T
In this case c=is the specific heat capacity for water (4.18kJ/mol) (Lloyd and Davenport,
2010, p. 56).
Mass is calculated twice thus the mass uncertainty is ±0.002
Percentage Uncertainty= Absolute Uncertainty
Value ∗100 %
For temperature
Absolute uncertainty= ±0.5
Percentage uncertainty
Percentage Uncertainty= Temperature Difference Error
Value ∗100 %
The uncertainties for mass and temperature can be summarized in the below table.
Mass Diff
Mean (g)
± 0.001
% mass
uncertainty
(%)
Temp Diff
mean
(oC)
± 0.5
%
temperatur
e
uncertainty
(%)
Total %
uncertainty
Methanol 4.4300 0.0451 79.50 1.257862 1.303
Ethanol 3.1125 0.0643 81.00 1.234568 1.299
Prop-1-ol 2.470 0.0810 78.00 1.282051 1.363
Prop-2-ol 3.0395 0.0658 79.00 1.265823 1.332
Butan-1-ol 2.6875 0.0744 78.00 1.282051 1.356
Pentan-1-ol 1.8905 0.106 53.50 1.869159 1.975
Hexan-1-ol 0.3530 0.567 53.00 1.886792 2.453
Considering the equation used to calculate energy
E=mc ∆ T
In this case c=is the specific heat capacity for water (4.18kJ/mol) (Lloyd and Davenport,
2010, p. 56).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
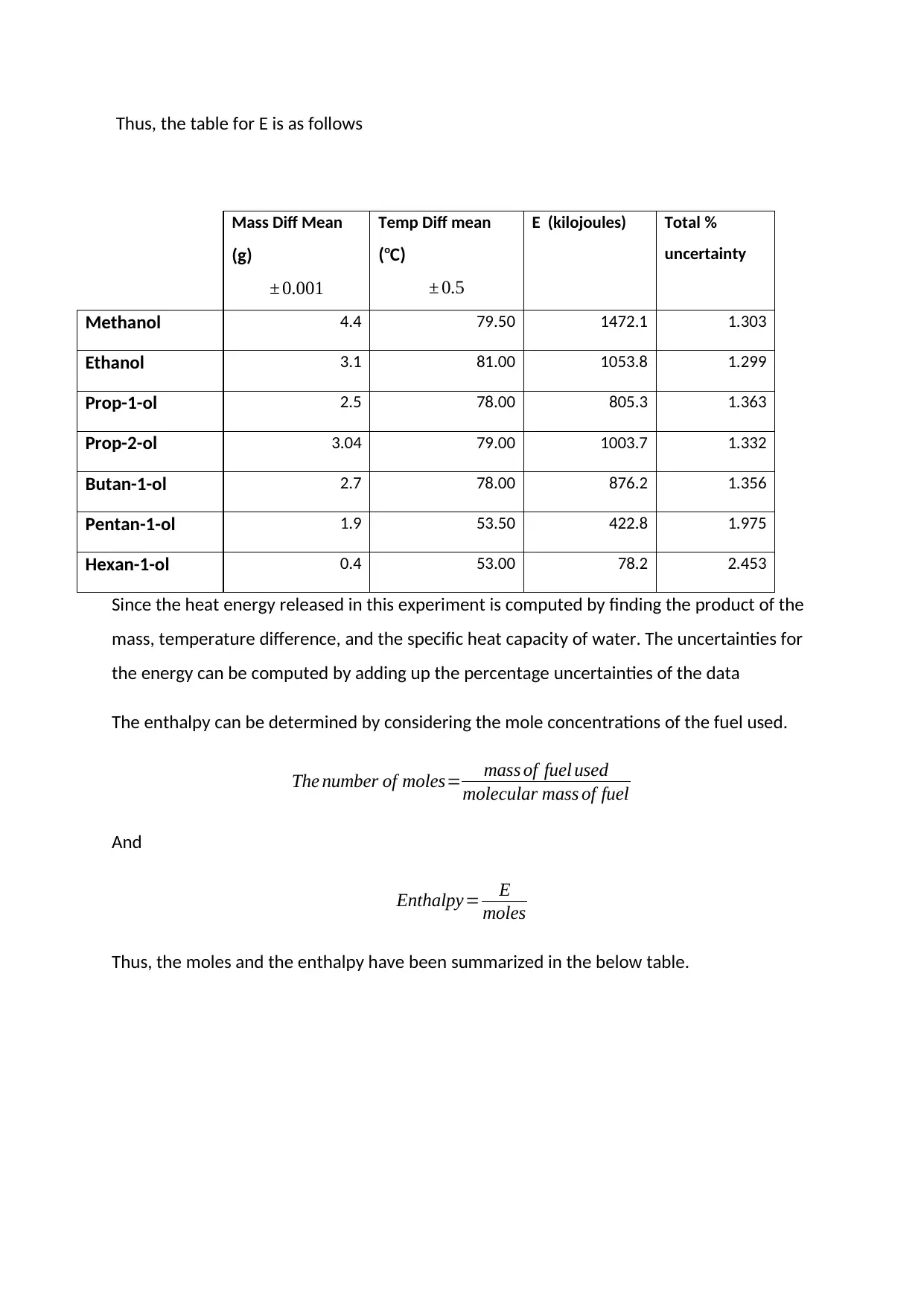
Thus, the table for E is as follows
Mass Diff Mean
(g)
± 0.001
Temp Diff mean
(oC)
± 0.5
E (kilojoules) Total %
uncertainty
Methanol 4.4 79.50 1472.1 1.303
Ethanol 3.1 81.00 1053.8 1.299
Prop-1-ol 2.5 78.00 805.3 1.363
Prop-2-ol 3.04 79.00 1003.7 1.332
Butan-1-ol 2.7 78.00 876.2 1.356
Pentan-1-ol 1.9 53.50 422.8 1.975
Hexan-1-ol 0.4 53.00 78.2 2.453
Since the heat energy released in this experiment is computed by finding the product of the
mass, temperature difference, and the specific heat capacity of water. The uncertainties for
the energy can be computed by adding up the percentage uncertainties of the data
The enthalpy can be determined by considering the mole concentrations of the fuel used.
The number of moles= mass of fuel used
molecular mass of fuel
And
Enthalpy= E
moles
Thus, the moles and the enthalpy have been summarized in the below table.
Mass Diff Mean
(g)
± 0.001
Temp Diff mean
(oC)
± 0.5
E (kilojoules) Total %
uncertainty
Methanol 4.4 79.50 1472.1 1.303
Ethanol 3.1 81.00 1053.8 1.299
Prop-1-ol 2.5 78.00 805.3 1.363
Prop-2-ol 3.04 79.00 1003.7 1.332
Butan-1-ol 2.7 78.00 876.2 1.356
Pentan-1-ol 1.9 53.50 422.8 1.975
Hexan-1-ol 0.4 53.00 78.2 2.453
Since the heat energy released in this experiment is computed by finding the product of the
mass, temperature difference, and the specific heat capacity of water. The uncertainties for
the energy can be computed by adding up the percentage uncertainties of the data
The enthalpy can be determined by considering the mole concentrations of the fuel used.
The number of moles= mass of fuel used
molecular mass of fuel
And
Enthalpy= E
moles
Thus, the moles and the enthalpy have been summarized in the below table.
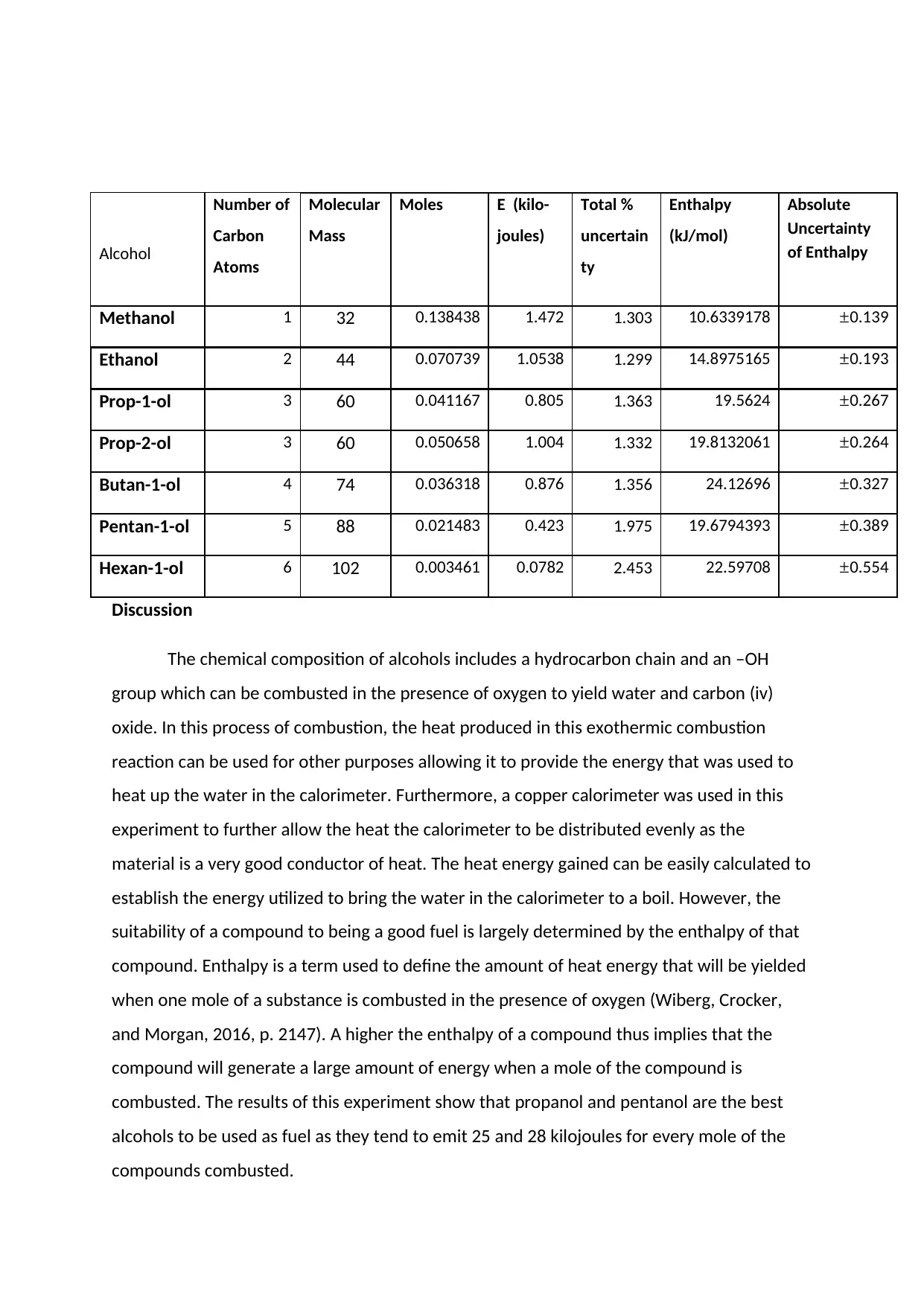
Alcohol
Number of
Carbon
Atoms
Molecular
Mass
Moles E (kilo-
joules)
Total %
uncertain
ty
Enthalpy
(kJ/mol)
Absolute
Uncertainty
of Enthalpy
Methanol 1 32 0.138438 1.472 1.303 10.6339178 ±0.139
Ethanol 2 44 0.070739 1.0538 1.299 14.8975165 ±0.193
Prop-1-ol 3 60 0.041167 0.805 1.363 19.5624 ±0.267
Prop-2-ol 3 60 0.050658 1.004 1.332 19.8132061 ±0.264
Butan-1-ol 4 74 0.036318 0.876 1.356 24.12696 ±0.327
Pentan-1-ol 5 88 0.021483 0.423 1.975 19.6794393 ±0.389
Hexan-1-ol 6 102 0.003461 0.0782 2.453 22.59708 ±0.554
Discussion
The chemical composition of alcohols includes a hydrocarbon chain and an –OH
group which can be combusted in the presence of oxygen to yield water and carbon (iv)
oxide. In this process of combustion, the heat produced in this exothermic combustion
reaction can be used for other purposes allowing it to provide the energy that was used to
heat up the water in the calorimeter. Furthermore, a copper calorimeter was used in this
experiment to further allow the heat the calorimeter to be distributed evenly as the
material is a very good conductor of heat. The heat energy gained can be easily calculated to
establish the energy utilized to bring the water in the calorimeter to a boil. However, the
suitability of a compound to being a good fuel is largely determined by the enthalpy of that
compound. Enthalpy is a term used to define the amount of heat energy that will be yielded
when one mole of a substance is combusted in the presence of oxygen (Wiberg, Crocker,
and Morgan, 2016, p. 2147). A higher the enthalpy of a compound thus implies that the
compound will generate a large amount of energy when a mole of the compound is
combusted. The results of this experiment show that propanol and pentanol are the best
alcohols to be used as fuel as they tend to emit 25 and 28 kilojoules for every mole of the
compounds combusted.
Number of
Carbon
Atoms
Molecular
Mass
Moles E (kilo-
joules)
Total %
uncertain
ty
Enthalpy
(kJ/mol)
Absolute
Uncertainty
of Enthalpy
Methanol 1 32 0.138438 1.472 1.303 10.6339178 ±0.139
Ethanol 2 44 0.070739 1.0538 1.299 14.8975165 ±0.193
Prop-1-ol 3 60 0.041167 0.805 1.363 19.5624 ±0.267
Prop-2-ol 3 60 0.050658 1.004 1.332 19.8132061 ±0.264
Butan-1-ol 4 74 0.036318 0.876 1.356 24.12696 ±0.327
Pentan-1-ol 5 88 0.021483 0.423 1.975 19.6794393 ±0.389
Hexan-1-ol 6 102 0.003461 0.0782 2.453 22.59708 ±0.554
Discussion
The chemical composition of alcohols includes a hydrocarbon chain and an –OH
group which can be combusted in the presence of oxygen to yield water and carbon (iv)
oxide. In this process of combustion, the heat produced in this exothermic combustion
reaction can be used for other purposes allowing it to provide the energy that was used to
heat up the water in the calorimeter. Furthermore, a copper calorimeter was used in this
experiment to further allow the heat the calorimeter to be distributed evenly as the
material is a very good conductor of heat. The heat energy gained can be easily calculated to
establish the energy utilized to bring the water in the calorimeter to a boil. However, the
suitability of a compound to being a good fuel is largely determined by the enthalpy of that
compound. Enthalpy is a term used to define the amount of heat energy that will be yielded
when one mole of a substance is combusted in the presence of oxygen (Wiberg, Crocker,
and Morgan, 2016, p. 2147). A higher the enthalpy of a compound thus implies that the
compound will generate a large amount of energy when a mole of the compound is
combusted. The results of this experiment show that propanol and pentanol are the best
alcohols to be used as fuel as they tend to emit 25 and 28 kilojoules for every mole of the
compounds combusted.

Further, in the case of propan-1ol and propan-2-ol, the latter is seen to yield a higher
enthalpy than the former regardless of the two compounds having the same molecular mass
and number of carbons. This observation can be attributed to the fact that the propan-1-ol
heated the water to boiling point faster than propan-2-ol. This implies that the molecular
structure of propan-2-ol has a structure that is different from that of propan-1-ol in terms of
the bonds and van der Waals forces that the two alcohols have. While enthalpy is
determined by the types and number of atoms that make up the molecular composition of
the compound, the molecular structure of the compound also plays very critical but
secondary role.
The errors that were experienced during this experiment were caused by the changes in the
environment of the experiment as temperature and pressure dissipation are not easy to
control. There might have also been some systematic errors associated with reading the
apparatus and carrying out the experiment as was required to the letter. Finally, errors
could also have resulted from random errors that occurs as the fuel was allowed to
evaporate during measurement of the weight resulting in errors (Rakopoulos, Rakopoulos,
Papagiannakis, and Kyritsis, 2011, p.1856). These errors can be reduced by completely
controlling the experiment through insulating it and placing air foams around the setup of
the experiment to prevent the high rates of heat loss.
Evaluation
The results I obtained show a trend in the enthalpies obtained as can be summarized in the
below graphs.
enthalpy than the former regardless of the two compounds having the same molecular mass
and number of carbons. This observation can be attributed to the fact that the propan-1-ol
heated the water to boiling point faster than propan-2-ol. This implies that the molecular
structure of propan-2-ol has a structure that is different from that of propan-1-ol in terms of
the bonds and van der Waals forces that the two alcohols have. While enthalpy is
determined by the types and number of atoms that make up the molecular composition of
the compound, the molecular structure of the compound also plays very critical but
secondary role.
The errors that were experienced during this experiment were caused by the changes in the
environment of the experiment as temperature and pressure dissipation are not easy to
control. There might have also been some systematic errors associated with reading the
apparatus and carrying out the experiment as was required to the letter. Finally, errors
could also have resulted from random errors that occurs as the fuel was allowed to
evaporate during measurement of the weight resulting in errors (Rakopoulos, Rakopoulos,
Papagiannakis, and Kyritsis, 2011, p.1856). These errors can be reduced by completely
controlling the experiment through insulating it and placing air foams around the setup of
the experiment to prevent the high rates of heat loss.
Evaluation
The results I obtained show a trend in the enthalpies obtained as can be summarized in the
below graphs.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
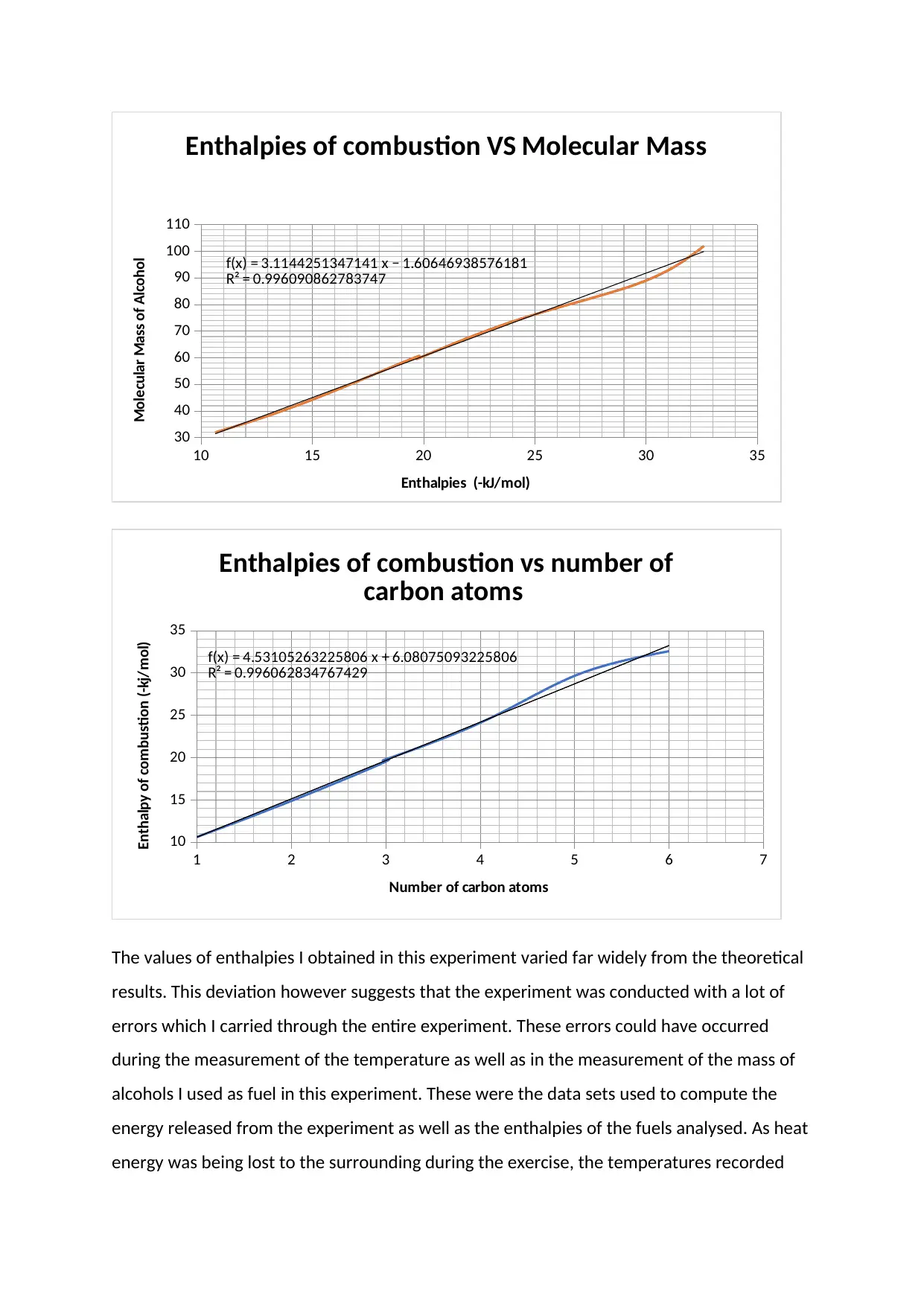
10 15 20 25 30 35
30
40
50
60
70
80
90
100
110
f(x) = 3.1144251347141 x − 1.60646938576181
R² = 0.996090862783747
Enthalpies of combustion VS Molecular Mass
Enthalpies (-kJ/mol)
Molecular Mass of Alcohol
1 2 3 4 5 6 7
10
15
20
25
30
35
f(x) = 4.53105263225806 x + 6.08075093225806
R² = 0.996062834767429
Enthalpies of combustion vs number of
carbon atoms
Number of carbon atoms
Enthalpy of combustion (-kj/mol)
The values of enthalpies I obtained in this experiment varied far widely from the theoretical
results. This deviation however suggests that the experiment was conducted with a lot of
errors which I carried through the entire experiment. These errors could have occurred
during the measurement of the temperature as well as in the measurement of the mass of
alcohols I used as fuel in this experiment. These were the data sets used to compute the
energy released from the experiment as well as the enthalpies of the fuels analysed. As heat
energy was being lost to the surrounding during the exercise, the temperatures recorded
30
40
50
60
70
80
90
100
110
f(x) = 3.1144251347141 x − 1.60646938576181
R² = 0.996090862783747
Enthalpies of combustion VS Molecular Mass
Enthalpies (-kJ/mol)
Molecular Mass of Alcohol
1 2 3 4 5 6 7
10
15
20
25
30
35
f(x) = 4.53105263225806 x + 6.08075093225806
R² = 0.996062834767429
Enthalpies of combustion vs number of
carbon atoms
Number of carbon atoms
Enthalpy of combustion (-kj/mol)
The values of enthalpies I obtained in this experiment varied far widely from the theoretical
results. This deviation however suggests that the experiment was conducted with a lot of
errors which I carried through the entire experiment. These errors could have occurred
during the measurement of the temperature as well as in the measurement of the mass of
alcohols I used as fuel in this experiment. These were the data sets used to compute the
energy released from the experiment as well as the enthalpies of the fuels analysed. As heat
energy was being lost to the surrounding during the exercise, the temperatures recorded

were lower than the expected values (Qi, et al., 2011, p. 1679). The lost heat energy can be
incorporated or considered by calculating the uncertainty level of the results from the
manner in which the important parameters were arrived at.
While the random errors in the experiment were low as has been seen in the experiment
due to a low possibilities error of the apparatus used, there was a big difference from the
literature values that are used as the enthalpy of combustion values for these alcohols. This
large difference can be attributed to the heat losses during the experiment and any
apparent loss of mass that might have occurred in the course of the experiment. This large
amount of heat lost during the experiment was the greatest weakness for this experiment.
Conclusion
The study has demonstrated that the heat released after the combustion of one
mole of a compound, increases with the increase in the number of carbon atoms in the
length of the hydrocarbon chain. This can be explained by the fact that an increase in the
number of carbon atoms in a hydrocarbon chain tends to increase the size of the fuel
particle. An increase in the carbon atoms translates into an increase in the molecular mass
of the compound which in turn affects the van der Waal’s forces and molecular bonds
joining the long chains. This is depicted in the graph that was obtained after the results
were plotted. This increase however was not observed to be a linear one.
Further Investigation
When I will be conducting further investigations, the impact of heat losses from the burner
and from the calorimeter ought to be considered. Further insulating the experiment is also
recommended to ensure that the heat losses have been completely reduced. The heat
losses can for instance be minimized through insulating the experiment surrounding using
an air foam around the experiment set up and through insulating the copper calorimeter.
The alcohols will also have to be burnt in the presence of oxygen to ensure that they are
fully combusted for more accurate results an even accurate inference of the experiment as
more energy will be release during complete combustion. The thermometer I used in the
experiment can also be substituted with an electronic thermometer that will enable the
errors that I encountered to be reduced a great deal due to the precision of reading the
incorporated or considered by calculating the uncertainty level of the results from the
manner in which the important parameters were arrived at.
While the random errors in the experiment were low as has been seen in the experiment
due to a low possibilities error of the apparatus used, there was a big difference from the
literature values that are used as the enthalpy of combustion values for these alcohols. This
large difference can be attributed to the heat losses during the experiment and any
apparent loss of mass that might have occurred in the course of the experiment. This large
amount of heat lost during the experiment was the greatest weakness for this experiment.
Conclusion
The study has demonstrated that the heat released after the combustion of one
mole of a compound, increases with the increase in the number of carbon atoms in the
length of the hydrocarbon chain. This can be explained by the fact that an increase in the
number of carbon atoms in a hydrocarbon chain tends to increase the size of the fuel
particle. An increase in the carbon atoms translates into an increase in the molecular mass
of the compound which in turn affects the van der Waal’s forces and molecular bonds
joining the long chains. This is depicted in the graph that was obtained after the results
were plotted. This increase however was not observed to be a linear one.
Further Investigation
When I will be conducting further investigations, the impact of heat losses from the burner
and from the calorimeter ought to be considered. Further insulating the experiment is also
recommended to ensure that the heat losses have been completely reduced. The heat
losses can for instance be minimized through insulating the experiment surrounding using
an air foam around the experiment set up and through insulating the copper calorimeter.
The alcohols will also have to be burnt in the presence of oxygen to ensure that they are
fully combusted for more accurate results an even accurate inference of the experiment as
more energy will be release during complete combustion. The thermometer I used in the
experiment can also be substituted with an electronic thermometer that will enable the
errors that I encountered to be reduced a great deal due to the precision of reading the

temperatures. If I had more time the experiment would have been conducted in a
controlled surrounding such that the impact of air pressure and temperature are minimized
to the very least.
References
Anderson, R.B., 2016. Thermodynamics of the hydrogenation of oxides of carbon. The
Journal of Physical Chemistry, 90(20), pp.4806-4810.
Bruno, T.J. and Smith, B.L., 2016. Enthalpy of combustion of fuels as a function of distillate
cut: application of an advanced distillation curve method. Energy & fuels, 20(5), pp.2109-
2116.
G., JAkarta. (2011, March). Chemistry Lab Report. Retrieved March 1, 2019, from
http://www.gandhijkt.org/blog/wp-content/uploads/2011/03/chemistry-sample-lab-
report.pdf
Lloyd, W.G. and Davenport, D.A., 2010. Applying thermodynamics to fossil fuels: Heats of
combustion from elemental compositions. Journal of chemical education, 57(1), p.56.
Qi, D.H., Chen, H., Geng, L.M., Bian, Y.Z. and Ren, X.C., 2010. Performance and combustion
characteristics of biodiesel–diesel–methanol blend fuelled engine. Applied Energy, 87(5),
pp.1679-1686.
controlled surrounding such that the impact of air pressure and temperature are minimized
to the very least.
References
Anderson, R.B., 2016. Thermodynamics of the hydrogenation of oxides of carbon. The
Journal of Physical Chemistry, 90(20), pp.4806-4810.
Bruno, T.J. and Smith, B.L., 2016. Enthalpy of combustion of fuels as a function of distillate
cut: application of an advanced distillation curve method. Energy & fuels, 20(5), pp.2109-
2116.
G., JAkarta. (2011, March). Chemistry Lab Report. Retrieved March 1, 2019, from
http://www.gandhijkt.org/blog/wp-content/uploads/2011/03/chemistry-sample-lab-
report.pdf
Lloyd, W.G. and Davenport, D.A., 2010. Applying thermodynamics to fossil fuels: Heats of
combustion from elemental compositions. Journal of chemical education, 57(1), p.56.
Qi, D.H., Chen, H., Geng, L.M., Bian, Y.Z. and Ren, X.C., 2010. Performance and combustion
characteristics of biodiesel–diesel–methanol blend fuelled engine. Applied Energy, 87(5),
pp.1679-1686.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Rakopoulos, D.C., Rakopoulos, C.D., Papagiannakis, R.G. and Kyritsis, D.C., 2011. Combustion
heat release analysis of ethanol or n-butanol diesel fuel blends in heavy-duty DI diesel
engine. Fuel, 90(5), pp.1855-1867.
Thornton, W.M., 2017. XV. The relation of oxygen to the heat of combustion of organic
compounds. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of
Science, 33(194), pp.196-203.
Wiberg, K.B., Crocker, L.S. and Morgan, K.M., 2011. Thermochemical studies of carbonyl
compounds. 5. Enthalpies of reduction of carbonyl groups. Journal of the American Chemical
Society, 113(9), pp.3447-3450.
heat release analysis of ethanol or n-butanol diesel fuel blends in heavy-duty DI diesel
engine. Fuel, 90(5), pp.1855-1867.
Thornton, W.M., 2017. XV. The relation of oxygen to the heat of combustion of organic
compounds. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of
Science, 33(194), pp.196-203.
Wiberg, K.B., Crocker, L.S. and Morgan, K.M., 2011. Thermochemical studies of carbonyl
compounds. 5. Enthalpies of reduction of carbonyl groups. Journal of the American Chemical
Society, 113(9), pp.3447-3450.
1 out of 14
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.





